0952
Assessment of Acute Kidney Injury and associated longitudinal changes with recovery using multiparametric renal MRI1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2Centre for Kidney Research and Innovation, University of Nottingham, Derby, United Kingdom
Synopsis
Acute kidney injury (AKI) is defined clinically using serum creatinine. We use multiparametric renal MRI to assess longitudinal changes in AKI. Nine participants were assessed at time of AKI, 7 were re-scanned at 3-months and 1-year. At peak AKI, total kidney volume (TKV) and cortex and medulla T1 were elevated, and cortex perfusion reduced compared to HVs. After 3-months, TKV reduced compared to peak AKI, cortex and medulla T1 remained slightly elevated compared to HVs. Perfusion remained reduced compared to HVs after 1-year. MRI showed incomplete recovery at 3 months, despite normalisation of biochemistry, providing potential to identify maladaptive repair.
INTRODUCTION
Acute Kidney Injury (AKI) is associated with marked increases in short and long term mortality, rates of subsequent chronic kidney disease (CKD) and risk of end stage kidney disease (ESKD).1 The severity of kidney injury and degree of recovery differs significantly between individuals, making it challenging to assess. Clinically, AKI is defined using changes in serum creatinine, a marker of non-specific renal excretory function.2 Multiparametric MRI offers the potential to assess and quantify pathophysiological processes relevant to AKI non-invasively without the use of gadolinium. Multiparametric MRI has been applied in humans to study CKD3,4 and renal transplantation,4, 5 but not to date in AKI. Mouse models of AKI6,7 show increased longitudinal relaxation time (T1), with greater changes in the medulla than cortex, and decreased cortical perfusion from day 7–28 following AKI, with these changes in T1 and perfusion differentiating AKI severity. Here we perform multiparametric MRI to assess renal pathophysiology in AKI and associated longitudinal changes.METHODS
Nine participants with AKI underwent an MRI scan as an in-patient at time of AKI, 7 participants attended follow-up scans at 3 months and 1 year following peak AKI (2 declined follow-up visits). In-patient MRI scanning was performed at a median of 6 days (IQR 5) after peak AKI (highest serum creatinine value). Scanning was performed on a 3T Philips Ingenia scanner. Coronal bTFE localiser scans were used for kidney volume measures. Arterial spin labelling (ASL) and T1 data were acquired using a spin-echo EPI readout in matched space (5 coronal-oblique slices, FOV 288x288mm2, resolution 3x3x5mm3, SENSE 2) using respiratory-triggered schemes. ASL comprised a flow alternating inversion recovery (FAIR) scheme (inflow map, post-label delay 1800ms, selective/non-selective thickness 45/400mm, 25 pairs), inversion recovery T1 data was acquired at 13 inversion times (200-1500ms). High resolution (1.5x1.5mm2) T1 measures were also obtained using a bFFE readout. T2* data was acquired with a 12 echo mFFE scheme (TE 5ms, echo spacing 3ms, 1.5x1.5x5mm3 and FOV 288x288mm2).Data Analysis: Total kidney volume (TKV) was calculated using Analyze9 and corrected for body surface area (BSA). Multiparametric maps were generated using Matlab. Inversion recovery data was fit to form T1 maps. Average perfusion weighted images were normalised to a base magnetisation image, and fit to a kinetic model to quantify perfusion. mFFE data was fit to compute T2* maps. Cortex and medulla masks were created from T1 maps, and the mode of all MRI parameters computed.
RESULTS
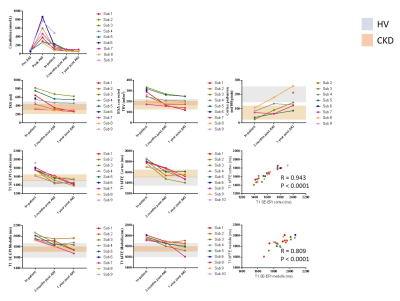
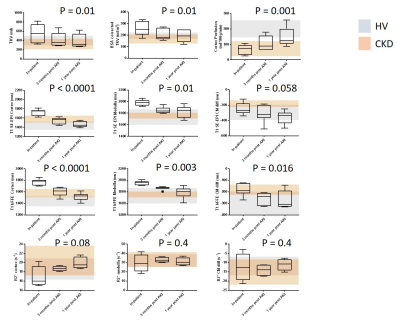
Serum creatinine levels for each participant show biochemical recovery in all at 1year post AKI (Figure 1). MRI measures for each of the AKI participants across the three visits are shown in Figure 1. Figure 2 shows the group data, with grey and orange bands indicating the range of values in healthy volunteer (HV) and CKD cohorts respectively.3 A repeated measures ANOVA showed a significant difference across visits for TKV (P = 0.01), cortex and medulla T1 (p < 0.0001 and p = 0.01), and cortex perfusion (P < 0.001).In-patient at time of AKI: TKV and BSA corrected TKV were elevated compared to HVs (p < 0.0001) and CKD (p < 0.0001). T1 of both the cortex and medulla were elevated compared to HV (cortex: p < 0.0001, medulla: p = 0.045) and CKD (cortex: p < 0.0001 medulla: p = 0.0006). Perfusion was reduced in comparison to HVs (p < 0.0001).
At 3 months post-AKI: TKV reduced in most participants to the HV range. Cortex T1 was elevated compared to HVs (p = 0.006) falling within the CKD patient range, whilst medulla T1 reduced to a lesser extent and remained elevated in comparison to CKD patients (p = 0.034). Perfusion increased compared to the in-patient scan (p = 0.02), but remained decreased compared to HVs (p = 0.002).
At 1 year post-AKI: TKV was unchanged from 3 months post-AKI. Cortex T1 was in the HV range for most subjects, whilst medulla T1 was at the upper range of HVs and CKD patients. Perfusion remained reduced 1 year post-AKI in most participants compared to HVs (p = 0.13).
BOLD changes were difficult to interpret because of associated changes in T2 due to inflammation.
DISCUSSION
AKI is associated with inflammation of the renal parenchyma8 with cell swelling and interstitial oedema. The pronounced increase in medulla and cortex T1 is consistent with this, with the medulla being particularly sensitive to changes at time of AKI, in agreement with animal models.9 Despite biochemical recovery in all patients there were still MR changes suggestive of reduced renal perfusion at 1yr post-AKI. Proteinuria resolution lagged behind improvement in creatinine but resolved by 1 year, unlike MRI measures, suggesting that the kidney remains vulnerable to further insults. This suggests a loss of renal reserve and therefore such patients may be more likely to have a further AKI episodes or progress to CKD.CONCLUSION
Serial creatinine alone does not give adequate information about renal recovery from AKI. MRI provides novel insights into the understanding of AKI, and can detect incomplete recovery at 3 months despite complete normalisation of biochemistry. MRI provides the potential to inform AKI aetiology, stratify severity and identify maladaptive repair, helping to develop future therapies.Acknowledgements
This work was funded by Animal Free Research UK. Animal Free Research UK is a UK medical research charity that funds and promotes non-animal techniques to replace animal experiments.References
1. Hoste, EAJ, Kellum, JA, Selby, NM, Zarbock, A, Palevsky, PM, Bagshaw, SM, Goldstein, SL, Cerda, J, Chawla, LS: Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol, 14: 607-625, 2018.
2. Selby, NM: A Comment on the Diagnosis and Definition of Acute Kidney Injury. Nephron, 141: 203-206, 2019.
3. Buchanan, C, Mahmoud, H, Cox, E, McCulloch, T, Prestwich, B, Taal, M, W., Selby, NM, Francis, ST: Quantitative assessment of structural and functional changes in CKD using multiparametric renal MRI and the association with biochemical and histological measures. Nephrol Dial Transplant, pii: gfz129. [Epub ahead of print], 2019.
4. Berchtold, L, Friedli, I, Crowe, LA, Martinez, C, Moll, S, Hadaya, K, de Perrot, T, Combescure, C, Martin, PY, Vallee, JP, de Seigneux, S: Validation of the corticomedullary difference in magnetic resonance imaging-derived apparent diffusion coefficient for kidney fibrosis detection: a cross-sectional study. Nephrol Dial Transplant, 2019.
5. Yu, YM, Ni, QQ, Wang, ZJ, Chen, ML, Zhang, LJ: Multiparametric Functional Magnetic Resonance Imaging for Evaluating Renal Allograft Injury. Korean J Radiol, 20: 894-908, 2019.
6. Hueper K, Peperhove M, Rong S, et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur. Radiol. 2014;24:2252–60.
7. Hueper K, Gutberlet M, Rong S, et al. Acute Kidney Injury: Arterial Spin Labeling to Monitor Renal Perfusion Impairment in Mice—Comparison with Histopathologic Results and Renal Function. Radiology, 2013;270:117-124
8. Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J Am Soc Nephrol. 2016;27(2):371-9.
9. Langenberg, C., et al. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med 2007;33(9): 1614-1618.
Figures