0936
Resting-state functional connectivity alterations in periventricular nodular heterotopia related epilepsy1Huaxi MR Research Center (HMRRC), West China Hospital of Sichuan University, Chengdu, China, 2Department of Neurology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
We performed the first resting-state fMRI study integrating both whole-brain functional connectivity (FC) and seed-based FC analyses to explore the network-level neural function alterations in patients with periventricular nodular heterotopia (PNH). Our findings (i) identified lower functional connectivity strength (FCS, an index of whole-brain connectivity) in bilateral insula, higher FC in the precuneus and lower FC in the anterior cingulate cortex/medial prefrontal cortex and cerebellum networks in PNH patients and (ii) demonstrated that the significant insular hypoactivation represented the cortical hub of the whole-brain networks in PNH, which might be of clinical significance in predicting disability progression of PNH.
Introduction
Periventricular nodular heterotopia (PNH) is a common structural malformation of cortical development in which nodules of neurons are ectopically retained along the lateral ventricles [1]. The clinical manifestations of PNH is characterized by seizure disorder ranging from mild to intractable, mental retardation, hypotonia and reading dysfluency [2]. Previous researches using diffusion MRI or structural MRI have revealed white matter disruption of the corpus callosum in PNH patients compared with controls [3, 4] and demonstrated that the strength of abnormal structural connectivity is higher among PNH patients with the longest duration of epilepsy [5]. However, the involvement of functional connectivity (FC) alterations in the pathogenesis of PNH remained elusive. Therefore, the aim of the current study was to explore the resting-state network-level neural function alterations in patients with PNH by integrating both whole-brain FC and seed-based FC analytical approaches [6]. Specifically, we firstly evaluated abnormalities in whole-brain FC, as measured by functional connectivity strength (FCS) [7], in PNH patients compared with controls. Additionally, regions with significant FCS alterations were selected as seeds in subsequent seed-based FC analyses [8]. Finally, we investigated the associations between these functional neural substrates and clinical features in PNH patients.Methods
38 patients diagnosed with PNH related epilepsy were recruited from the epilepsy center of the West China Hospital while 38 healthy controls (HCS) matched for age and gender were enrolled through posters. This investigation was approved by the local ethical committee and all subjects had provided informed consent. Assessment of mental state was performed by Mini-mental State Examination (MMSE). The 14-item Hamilton Anxiety Scale (HAMA) and 17-item Hamilton Depression Scale (HAMD) were used to rate anxiety and depressive symptoms, respectively. Resting-state fMRI data of all the participants were acquired via a 3-Tesla Siemens MRI system with an 8 channel phase array head coil (TR/TE=2000/30msec, flip angle=90°, slice thickness=5mm with no gap, 30 axial slices, 200 volumes in each run). The preprocessing of functional images was performed using DPABI software [9]. The calculation of FCS maps was performed using REST toolbox [10]. Afterwards, we chose the regions with FCS alterations in PNH compared with HCS as seeds to conduct additional seed-based interregional correlation analyses in order to explore more specific abnormalities of FC patterns related to the identified hubs. The statistical analyses of FCS and seed-based FC maps between PNH patients and HCS were conducted using the voxel-based two-sample t-test in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The voxel level statistical threshold was set at P < 0.001 with a minimum cluster extent of 100 voxels without correction. Meanwhile, the statistical threshold of cluster level was set at P < 0.05 with family-wise error (FWE) correction. Areas with significant FCS or FC alterations between groups were extracted as regions of interest for Pearson correlation analyses with clinical variables including MMSE scores, HAMA scores, HAMD scores, seizure frequency and duration of epilepsy.Results
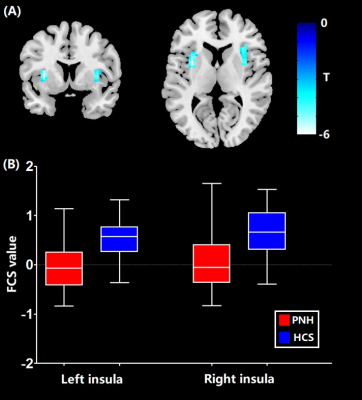
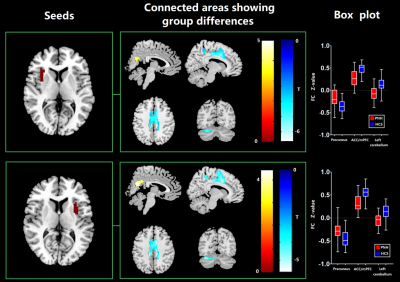
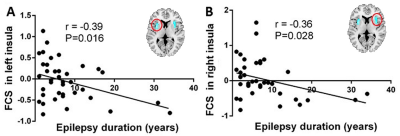
In comparison with controls, FCS reduction of bilateral insula was identified in PNH patients (P < 0.05, FWE correction) (Figure 1). Meanwhile, PNH patients showed higher FC in the precuneus and lower FC in the left cerebellum and anterior cingulate cortex (ACC) extending to medial PFC with seed placed in the left insula where higher FCS was detected (P<0.05, FWE correction). These findings of FC alterations remained reproducible when the seed was placed in the right insula (P<0.05, FWE correction) (Figure 2). Pearson correlation analyses revealed that the epilepsy duration was negatively correlated with FCS value in the left insula (r = -0.39, P = 0.015) and right insula (r = -0.36, P = 0.028), separately.Discussion
In the current study, we found FCS reductions in the bilateral insular areas and higher FC in the precuneus in patients with PNH relative to controls. The insula is the core structure of the salience network (SN) which plays a key role in regulating the dynamic changes of external stimuli and internal events [11]. while the precuneus is the main component of the default mode network (DMN), which is involved in the monitoring of internal processes including autobiographical and self-monitoring [12]. The hypoactivation of the insula and the hyperactivation of precuneus suggested the disequilibrium between the DMN and the SN might be associated with the pathophysiology of PNH.Conclusion
Using the resting-state FCS analytical approach, we identified significant insular hypoactivation in PNH patients, which suggests that the insula might represent the cortical hub of the whole-brain networks in this condition. Meanwhile, Pearson correlation findings indicated the insular FCS might be of clinical significance in predicting disability progression in PNH patients. Additionally, the disruption of resting-state FC in the DMN and the fronto-limbic-cerebellar circuits pointed to a connectivity-based neuropathological process in PNH.Acknowledgements
This study was supported by National Nature Science Foundation (Grant NO. 81671669), Science and Technology Project of Sichuan Province (Grant NO. 2017JQ0001)References
[1].Mirandola, L., et al., Stereo-EEG: Diagnostic and therapeutic tool for periventricular nodular heterotopia epilepsies. Epilepsia, 2017. 58(11): p. 1962-1971.
[2]. Aghakhani, Y., et al., The role of periventricular nodular heterotopia in epileptogenesis. Brain, 2005. 128(Pt 3): p. 641-51.
[3].Farquharson, S., et al., Periventricular Nodular Heterotopia: Detection of Abnormal Microanatomic Fiber Structures with Whole-Brain Diffusion MR Imaging Tractography. Radiology, 2016. 281(3): p. 896-906.
[4]. Liu, W., et al., Integrity of the corpus callosum in patients with periventricular nodular heterotopia related epilepsy by FLNA mutation. Neuroimage Clin, 2018. 17: p. 109-114.
[5]. Christodoulou, J.A., et al., Abnormal structural and functional brain connectivity in gray matter heterotopia. Epilepsia, 2012. 53(6): p. 1024-32.
[6].Buckner, R.L., F.M. Krienen, and B.T. Yeo, Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci, 2013. 16(7): p. 832-7.
[7]. Buckner, R.L., et al., Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci, 2009. 29(6): p. 1860-73.
[8].Biswal, B., et al., Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med, 1995. 34(4): p. 537-41.
[9].Yan, C.G., et al., DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics, 2016. 14(3): p. 339-51.
[10].Song, X.W., et al., REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 2011. 6(9): p. e25031.
[11].Menon, V. and L.Q. Uddin, Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct, 2010. 214(5-6): p. 655-67.
[12].Raichle, M.E., The brain's default mode network. Annu Rev Neurosci, 2015. 38: p. 433-47.
Figures