0931
White matter microstructure characterisation in left and right temporal lobe epilepsy (TLE) using TBSS1Department of Brain and Behavioral Science, University of Pavia, Pavia, Italy, 2Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta, Milan, Italy, 3Neuroradiology, Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta, Milan, Italy, 4Neuroradiology Unit, Brain MRI 3T Research Center, IRCCS Mondino Foundation, Pavia, Italy, 5Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, NMR Research Unit, Queen Square MS Centre, London, United Kingdom, 6Brain MRI 3T Research Center, IRCCS Mondino Foundation, Pavia, Italy
Synopsis
Tract-based spatial statistics investigations of temporal lobe epilepsy (TLE) have been using standard diffusion metrics, without distinguishing patients according to the lateralization of their epileptogenic zone. The aim of this study is to further our knowledge by identifying specific patterns of alteration in left and right TLE patients using diffusion kurtosis imaging and NODDI parameter maps. Our findings demonstrate the presence of specific patterns of white matter alterations, with the left TLE more widely affecting both cerebral and cerebellar regions. These results support the need to consider patients separately, according to the side of their pathology.
Introduction
General white matter (WM) alterations in Temporal Lobe Epilepsy (TLE) are well documented [1]. Diffusion tensor imaging (DTI) has been used to study WM abnormalities. Diffusion kurtosis imaging (DKI) [2] and neurite orientation dispersion and density imaging (NODDI) [3], have been proposed as advanced methods to calculate metrics sensitive to microstructural alterations. Tract-based spatial statistics (TBSS) [4] is a fully automated technique for voxel-wise statistics on diffusion metrics while minimizing possible misalignment between subjects.Here, TBSS was applied to DTI, DKI and NODDI metrics calculated on patients with drug-resistant TLE in order (i) to investigate patterns of WM alterations for left and right TLE patients, and (ii) to explore the sensitivity of multiple diffusion metrics to microstructural changes.
Methods
Subjects54 TLE patients (35.6±10.5y, 24 males) and 36 healthy controls (HC) (35.6±8.4y, 19 males) were considered. Seizures were lateralized according to medical history, neurologic examination, interictal electroencephalography, and positron emission tomography. Twenty-seven patients had the epileptic focus in the left hemisphere (32.2±10.7y, 13 males) while 27 had right TLE (38.9±9.4y, 11 males).
MRI acquisition & analysis
Acquisition: MRI data were acquired with a 3T Siemens Skyra (Siemens AG, Erlangen, Germany) using clinical and advanced sequences. Diffusion weighted (DW) images were acquired with a twice refocused SE-EPI sequence, TR/TE=8400/93ms, 70 axial slices with no gap, 2.2mm isotropic voxel, 45 volumes repeated for two-shell non-collinear DW directions with b=1000/2000s/mm2 and 9 volumes with no DW (b=0s/mm2).
Preprocessing: gibbs ringing reduction, denoising, eddy-currents and geometrical distortion correction, and motion realignment were performed on the diffusion images (FSL, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki).
DW analysis: DTI was fitted and maps of fractional anisotropy (FA), mean diffusivity (MD), axial and radial diffusivities (AD and RD) were created; DKI provided mean, axial and radial kurtosis (mK, aK and rK); NODDI provided maps of orientation dispersion index (ODI) and neurite density index (NDI).
TBSS: The best target was identified among the subjects, then TBSS was performed with default options. [https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide]
Statistics: Voxelwise statistics were performed on the skeletonised data (covariates: age and gender). For each metric, differences (p=0.05 with correction for multiple comparisons) were evaluated between: TLEall-HC, TLEleft-HC, TLEright-HC. Normality test of clinical variables was performed using SPSS (v.21) (IBM, Armonk, NewYork).
Results
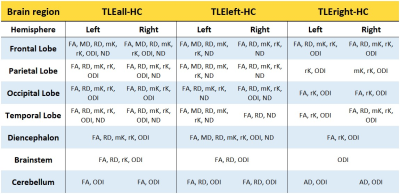
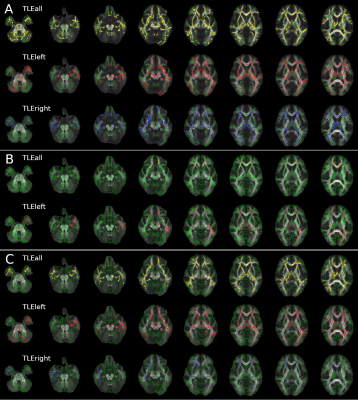
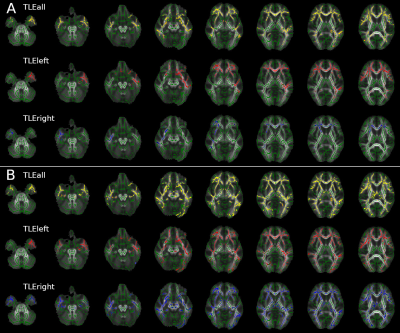
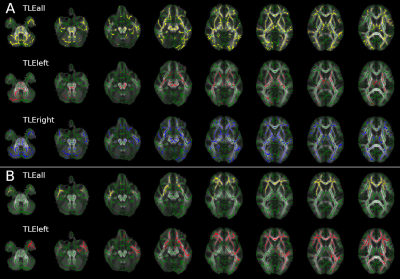
Widespread alterations were found in all diffusion metrics, other than AD and aK, in TLE patients compared to HC (Table1).Left TLE: Bilateral FA and RD alterations were found in cerebral and cerebellar WM with the exclusion of the right temporal lobe (less widespread). MD alterations were predominantly found in the left hemisphere and involved temporal, diencephalon, parietal and frontal lobe (Figure1). Kurtosis maps (mK and rK) revealed widespread brain alterations with the exclusion of the cerebellum, brainstem and a large portion of the right temporal lobe (Figure2). ODI showed widespread alterations of the cerebellum (worst in the right side), diencephalon, brainstem and right occipital lobe (optic radiation). No changes were detected in temporal lobes other than in the inferior longitudinal fasciculus. ND alterations spread all over the brain, more consistently in the left hemisphere, with the exception of brainstem and cerebellum (Figure3).
Right TLE: Bilateral FA alterations were found in temporal, frontal and occipital lobes and diencephalon. AD alterations affected the cerebellar peduncles; RD alterations were mainly found in the right temporal and a few in the frontal lobe plus the corpus callosum (Figure1). Alterations in the right temporal lobe and bilaterally in the frontal lobe were revealed by mK, while rK showed widespread alterations with the exclusion of the cerebellum and lower area of the brainstem (Figure2). ODI alterations were spread throughout the brain (Figure3).
Discussion & Conclusions
Our findings revealed that TLE patients have patterns of microstructural alterations reflecting the side of their epileptogenic zone. Indeed, most of the DW metrics demonstrated alterations between TLE patients and HC. These widespread alterations, though, could mislead interpretations because they are overall results from specific changes typical of different pathological substrates. This statement is supported by our findings of left and right TLE differences with HC. Indeed, these two clinical manifestations presented different patterns of alterations, characterized by specific microstructural alterations involving different brain regions. DTI and DKI indexes demonstrated that left TLE patients are characterized by more extensive and generalized damage of WM areas with respect to HC. In particular, all metrics revealed several bilateral regions of abnormalities. Right TLE patients, instead, showed more focal and side-specific alterations for all diffusion metrics suggesting a more localised pathological involvement. It is worth noting that the cerebellum, often not even mentioned in TBSS works, was differently involved in left and right TLE patients: FA reduction and RD increase characterized the whole cerebellum of left TLE patients, while none of the considered metrics was detecting cerebellar alterations in right TLE patients.Acknowledgements
3TLE is a multicentric research project granted by Italian Health Ministry (NET2013-02355313): Magnetic resonance imaging in drug-refractory temporal lobe epilepsy: standardization of advanced structural and functional protocols at 3T, to identify hippocampal and extra-hippocampal abnormalities.
Acknowledgments to the UCL-UCLH Biomedical Research Centre for ongoing funding; the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634541. Spinal Research (UK), Wings for Life (Austria), Craig H. Neilsen Foundation (USA) (jointly funding the INSPIRED study), Wings for Life (#169111), the UK Multiple Sclerosis Society (grants 892/08 and 77/2017).
References
1 Liu Z, Xu Y, An J, et al. (2015) Altered Brain White Matter Integrity in Temporal Lobe Epilepsy: A TBSS Study. J Neuroimaging 25:460–464. doi: 10.1111/jon.12154
2 Veraart J, Novikov DS, Christiaens D, et al. (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. doi: 10.1016/j.neuroimage.2016.08.016.Denoising
3 Zhang H, Schneider T, Wheeler-Kingshott CAM, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–16. doi: 10.1016/j.neuroimage.2012.03.072
4 S.M. Smith, M. Jenkinson, H. Johansen-Berg, D. Rueckert, T.E. Nichols, C.E. Mackay, K.E. Watkins, O. Ciccarelli, M.Z. Cader, P.M. Matthews, and T.E.J. Behrens. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31:1487-1505, 2006.
Figures