0926
Abnormal static and dynamic functional connectivity in active professional fighters with repetitive head trauma: A resting-state fMRI study1Lou Ruvo Center for Brain Health, Cleveland Clinic, Las Vegas, NV, United States, 2Department of Neuroscience, University of California, San Diego, La Jolla, CA, United States, 3Neuroscience Institute, Hoag Hospital, Irvine, CA, United States, 4University of Colorado, Boulder, Boulder, CO, United States
Synopsis
Both static and dynamic functional connectivity differences between cognitively impaired and non-impaired active professional fighters were first explored. Significant decreased static functional connections and trend-level increased dynamic functional connections among regions involved in memory and executive functions were found in cognitively impaired fighters, which adds brain functional reorganizations to previously observed structural damages in brain deficits related to repetitive head trauma. We further demonstrated that both static and dynamic functional connectivity were sensitive to cognitive declines in this fighter’s cohort, as both static and dynamic functional features can reliably predict cognitive impairment status in fighters.
Introduction.
Repetitive exposures to head trauma is a risk factor for multiple neuro-degenerative disorders1,2. Active professional fighters experiencing repetitive head trauma in sanctioned competitions and daily trainings, are at risks of potential brain damages. Previous neuroimaging studies have identified abnormalities in brain regional volumes, cortical thickness and white-matter connections in active professional fighters, which correlate with their cognitive task performances3–5. In this study, in the same cohort of active professional fighters, we investigated brain functional changes using both static and dynamic functional connectivity analysis, which would provide supplementary functional knowledge to brain deficits caused by repetitive head trauma.Methods.
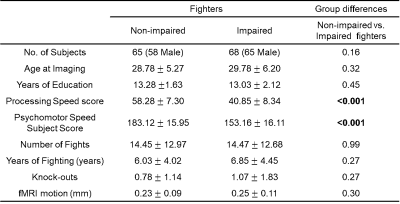
Subjects. 68 cognitively impaired fighters and 65 cognitively non-impaired fighters from the Professional Fighters Brain Health Study6 were included in the analysis. Fighters’ cognitive impairment status was predefined using neuropsychological tests. Processing speed (PSS) and psychomotor speed (PSY) were used to measure the cognitive impairment status and subject full demographics are listed in Table. 1. Resting-state fMRI data were collected for all subjects on a 3T Siemens scanner (TR/TE/resolution=2.8s/28ms/2x2x4mm3, 30 slices, axial acquisition, 137 time frames). A T1-weighted structural image was also acquired with a standard MPRAGE sequence. Static and dynamic functional connectivity (FC) matrix. Each T1 image was input to the FreeSurfer pipeline7 and a subject-specific anatomical segmentation was generated with 66 cortical regions of interest (ROIs) based on Desikan-Killiany8 atlas and 12 sub-cortical ROIs. This subject-specific parcellation was co-registered to fMRI native space using 12 parameters affine transformation. After standard preprocessing steps, average resting-state fMRI time series were obtained for 78 ROIs in each fighter. Static FC matrix was then computed using the Pearson’s correlation between average time series of every ROI-pair. Dynamic FC matrix was estimated using a sliding-window approach, with an optimum window-size computed at every time point9. Standard deviation over all windowed functional connectivity matrices was used to quantify the temporal dynamics for every ROI pair. Network-based statistical analysis. Network based statistics10 was used to compare static and dynamic FC matrix between cognitively impaired and non-impaired fighters, with age, gender, years of education and fMRI root-mean-square motion as covariates. A threshold of t=2.92 (puncorr=0.002) was applied to identify a set of supra-threshold connections which showed significant differences (pcorr<0.05) in network-based statistics. Classification. Both static and dynamic FC matrix were used as features to predict cognitive impairment status in fighters. An automated feature selection step and a non-linear classifier were included in the classification framework. A ten-fold cross validation was used to determine the classification accuracy and the ten-fold division was repeated 100 times to avoid division bias.Results.
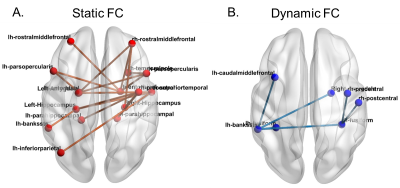
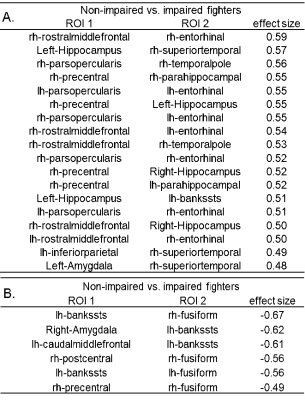
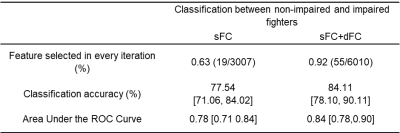
Static FC. 18 functional connections are significantly stronger (pcorr<0.05) in cognitively non-impaired fighters, as compared to cognitively impaired fighters (Fig. 1(A)). These paths comprise both cortical and subcortical brain regions, including inferior frontal gyrus, middle frontal gyrus, hippocampus, para-hippocampal gyrus, entorhinal cortex, amygdala, precentral gyrus, temporal pole, superior temporal gyrus, banks of superior temporal sulcus, and inferior parietal lobe. Most of these regions form connections essential to executive and memory functions. Medium effect sizes (Cohen’s d from 0.48 to 0.62) are observed for all connections (Table 2(A)). Dynamic FC. No statistical significant difference (pcorr<0.05) was observed between cognitively non-impaired and impaired fighters in the network-based statistics analysis. Only 6 paths showed a higher dynamic FC values in cognitively impaired fighters at the trend level with pcorr=0.09 (Fig. 1(B)). These connections depict higher temporal variations in impaired fighters corroborate with regions showing lower static functional connections, and all 6 paths had a medium effect sizes (Table 2 (B)). Classification. Static FC alone can reliably predict cognitive status in fighters with an accuracy 77.54% (Table 4). Adding dynamic FC to the feature set further improves the prediction accuracy by 8% and reaches 84.11%.Discussion.
Our study identified a set of significant decreased static functional connections and trend-level increased dynamic functional connections among regions involved in memory and executive functions in cognitively impaired fighters, as compared to cognitively nonimpaired fighters, which adds supplementary knowledge of functional brain reorganizations to previous structural findings related to repetitive head trauma. We further demonstrated that both static and dynamic FCs are sensitive to cognitive declines in this fighter’s cohort, as both static and dynamic FC features can reliably predict cognitive impairment status in fighters.Acknowledgements
The study is supported by the National Institutes of Health (grant number P20GM109025), a private grant from the Peter and Angela Dal Pezzo funds, a private grant from Lynn and William Weidner, a private grant from Stacie and Chuck Matthewson and the young scientist award at Cleveland Clinic Lou Ruvo Center for Brain Health (Keep Memory Alive Foundation). The PFBHS is supported by Belator, UFC, the August Rapone Family Foundation, Top Rank, and Haymon Boxing.References
1. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451. doi:10.1097/HTR.0b013e3181c15600
2. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. 2013;9(4):222-230. doi:10.1038/nrneurol.2013.33
3. Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: The Professional Fighters’ Brain Health Study. Br J Sports Med. 2015;49(15):1007-1011. doi:10.1136/bjsports-2014-093877
4. Mishra VR, Zhuang X, Sreenivasan KR, et al. Multimodal MR Imaging Signatures of Cognitive Impairment in Active Professional Fighters. Radiology. 2017;0(0):Ryan, J. O., Pakhomov, S. V. S., Marino, S., Berni. doi:10.1148/radiol.2017162403
5. Mishra VR, Sreenivasan KR, Zhuang X, et al. Understanding white matter structural connectivity differences between cognitively impaired and nonimpaired active professional fighters. Hum Brain Mapp. 2019;(March):hbm.24761. doi:10.1002/hbm.24761
6. Bernick C, Banks S, Phillips M, et al. Professional fighters brain health study: Rationale and methods. Am J Epidemiol. 2013;178(2):280-286. doi:10.1093/aje/kws456
7. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. doi:10.1016/j.neuroimage.2012.01.021
8. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi:10.1016/j.neuroimage.2006.01.021
9. Cordes D, Zhuang X, Kaleem M, et al. Advances in functional magnetic resonance imaging data analysis methods using Empirical Mode Decomposition to investigate temporal changes in early Parkinson’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:372-386. doi:10.1016/J.TRCI.2018.04.009
10. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53(4):1197-1207. doi:10.1016/j.neuroimage.2010.06.041
Figures