0921
Quantitative 23Na MRI of mild traumatic brain injury: Initial findings1Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Department of Rehabilitation Medicine, New York University School of Medicine, New York, NY, United States, 3Ronald O. Perelman Department of Emergency Medicine, New York University School of Medicine, New York, NY, United States, 4Department of Psychiatry, New York University School of Medicine, New York, NY, United States
Synopsis
In this quantitative sodium MRI study, 24 mild traumatic brain injury (mTBI) and 9 controls were scanned at 3 T. Total sodium content (TSC) was calculated in five different subcortical regions, as well as in global grey and white matter. TSC in mTBI did not differ statistically from controls for the examined regions. Patients with findings on conventional 1H imaging (e.g. lesions, microhemorrhages) did not differ from patients without such findings in their TSC. More patients and controls are being recruited to strengthen the statistical power of these comparisons.
Introduction
Traumatic brain injuries (TBI) secondary to head trauma are one of the main causes of neurological disabilities worldwide, particularly in young adults1. Despite its low severity, mild TBI often presents physical, psychiatric, emotional and cognitive disorders2, however the biological correlates of these manifestations are not detected on CT and MRI3. Cerebral 23Na MRI allows a quantitative assessment of new biochemical information in brain, such as ion homeostasis, which is vital for cell viability. This advanced technique has been used in recent years mainly in multiple sclerosis4 and migraine5. In TBI, white matter is susceptible to injury caused by acceleration, deceleration and rotational forces. The Na+/K+ exchange pump can be affected by energy deficits due to mitochondrial dysfunction, as well as by diffuse axonal injury, the histopathological signature of TBI6,7. The purpose of this study was to investigate the total sodium content (TSC) in different brain regions of patients with mTBI within one month of injury. TSC measures were correlated to standard imaging and clinical evaluation.Materials & Methods
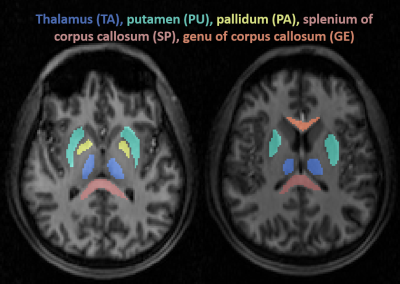
Twenty-four patients (18-60 years, 16 females) with confirmed mTBI, and nine age-matched healthy volunteers (23-54 years, 6 females) were recruited as controls for this study. Images were acquired 23±9.5 days after the injury on a 3T scanner (Magnetom Prisma, Siemens Healthineers). A 20-channel quadrature head coil (Siemens Healthineers) was used for 1H imaging. The qualitative screening included 3D MPRAGE (TR/TE/TI = 2400/2.24/1060 ms; flip angle = 8°; in plane FOV = 256x256 mm2; 208 slices, slab thickness = 0.8 mm; voxel size = 0.8×0.8x0.8 mm³; TA = 6:38 min), FLAIR (TR/TE/TI = 9000/81/2500 ms; 30 slices, in-plane FOV = 220x220 mm2; voxel size = 0.7×0.7×5.0 mm³; TA = 2:44 min) and SWI (TR/TE = 28/20 ms; flip angle = 15°; in-plane FOV = 220x220 mm2; voxel size = 0.7×0.7×3.0 mm³; TA = 3:46 min). A radiologist evaluated these images for possible lesions and microhemorrhages.Sodium imaging was performed using a 1H/23Na double-tuned head coil (in-house built). All 23Na images were acquired using a 3D ultrashort TE non-Cartesian FLORET sequence9. The spin-density weighted image was acquired with the following parameters: TE = 0.2 ms, TR = 100 ms, 3 hubs at 45°, number of interleaves/hub = 26, resolution = 6 mm isotropic, 46 averages, TA 5:59 min. 23Na signals were calibrated using the eyes. Brain segmentation was performed using SPM12 (UCL, UK) from MPRAGE data. In addition to global grey matter (GM) and white matter (WM), we estimated the TSC in different regions on the brain: thalamus (TH), putamen (PU), pallidum (PA), and the splenium (SP) and genu (GE) of the corpus callosum (see Figure 1).
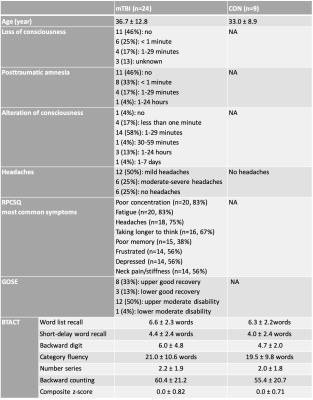
All TBI patients also underwent clinical and neurocognitive testing. The clinical examination included the Rivermead post-concussion symptoms questionnaire (RPQ)10, the ICHD-3 Headache screening11, and the Glasgow Outcome Scale – Extended (GOSE)12. For the neurocognitive assessment, we used the Brief Test of Adult Cognition by Telephone (BTACT) that addresses episodic memory and executive functioning13.
The two-sided Wilcoxon rank sum test was applied to the median of the TSC over the five different regions and the global GM and WM in all subjects to assess the significance of their difference between control and TBI. Statistical difference was defined as p<0.05.
Results
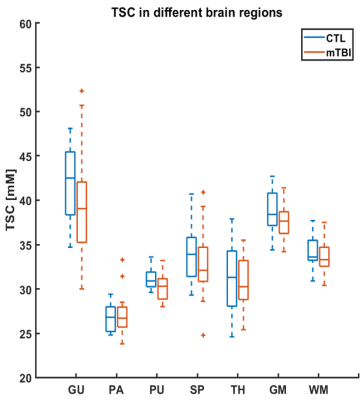
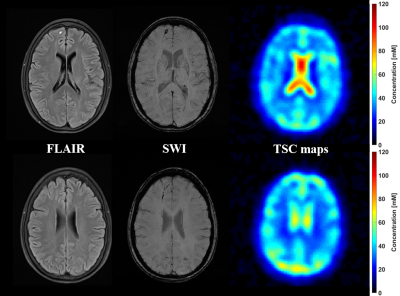
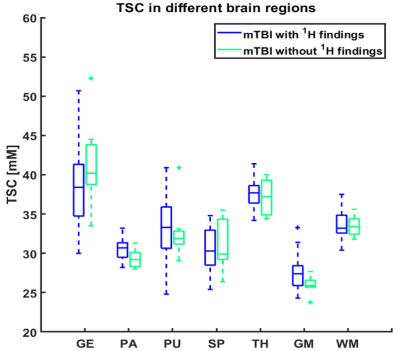
The demographics and cognitive measures are presented in Table 1. The individual tests and the composite z-scores of the BTACT did not differ between controls and mTBI. One patient had microbleeds, five patients had FLAIR signal abnormalities and one patient had an axonal shear injury with corresponding microhemorrhages. The presence of these findings did not correlate with the clinical and neurocognitive testing. There were no differences in TSC between mTBI and controls for the global GM and global WM nor for any of the subcortical regions (Figure 2). Five patients had TSC changes higher than two SD of the mean in at least one of the following regions: genu, splenium and pallidum.Figure 3 presents one example of TSC map with the corresponding FLAIR and SWI images. Patients with findings on conventional 1H imaging (e.g. lesions, microhemorrhages) did not differ from patients without such findings in their TSC (Figure 4). Both patient groups displayed a variety of outcome in the clinical and neurocognitive tests.
Discussion & Conclusion
We evaluated 23Na MRI for providing quantitative indices related to pathological changes after mild TBI. There were no statistically significant findings for global measurement of TSC in the examined regions of the brain. However, the median TSC values in mTBI were consistently lower compared to those in matched controls.The sample size of this patient cohort is being increased and is now being investigated with 1H and 23Na MRI three months and one year after the first exam to determine the relationship between head trauma indices and the clinical outcome of mTBI. As importantly, more controls are being added to increase the statistical power of the above comparisons.
Acknowledgements
This project was supported by grant R01NS097494 from the National Institute of Health (NIH).References
1. Dang, B., Chen, W., He, W. & Chen, G. Rehabilitation Treatment and Progress of Traumatic Brain Injury Dysfunction. Neural Plast. 2017, 1–6 (2017).
2. Maas, A. I. R. et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048 (2017).
3. Blennow, K. et al. Traumatic brain injuries. Nat. Rev. Dis. Prim. 2, 16084 (2016).
4. Petracca, M. et al. Brain intra- and extracellular sodium concentration in multiple sclerosis: a 7 T MRI study. Brain 139, 795–806 (2016).
5. Meyer, M. M. et al. Cerebral sodium (23Na) magnetic resonance imaging in patients with migraine — a case-control study. Eur. Radiol. (2019). doi:10.1007/s00330-019-06299-1
6. Saraiva, A. L. L. et al. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res. Bull. 87, 180–186 (2012).
7. Lima, F. D. et al. Na+,K+-ATPase activity impairment after experimental traumatic brain injury: Relationship to spatial learning deficits and oxidative stress. Behav. Brain Res. 193, 306–310 (2008).
8. Grover, H. et al. MRI Evidence of Altered Callosal Sodium in Mild Traumatic Brain Injury. Am. J. Neuroradiol. 39, 2200–2204 (2018).
9. Pipe, J. G. et al. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn. Reson. Med. 66, 1303–1311 (2011).
10. King, N. S., Crawford, S., Wenden, F. J., Moss, N. E. G. & Wade, D. T. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 (1995).
11. Vincent, M. & Wang, S. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38, 1–211 (2018).
12. Wilson, J. T., Pettigrew, L. E. & Teasdale, G. M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–85 (1998).
13. Tun, P. A. & Lachman, M. E. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing 35, 629–632 (2006).
Figures