0920
Quantitative 31P MRS Assessment of Neurometabolic Derangement in Pediatric Concussion1CMRR, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Children’s Minnesota Neuroscience Institute, St. Paul, MN, United States, 3Department of Neurology, University of Minnesota,, Minneapolis, MN, United States
Synopsis
Abnormal changes in brain metabolism and its role in pediatric concussion have not been well studied. We employed 31P MRS technique at 7T to assess the neurometabolic alteration in children with concussion. Phosphorous metabolites concentrations and other key physiological parameters were measured in patient and control cohorts. Metabolic differences between healthy and concussed brains were detected at two time points after the injury. We also found that mild head trauma reduced the age-dependences of high-energy phosphates and NAD contents in the developing brain, and it took much longer than clinically defined “recovery time” to fully restore such relationship.
INTRODUCTION
Concussion is a mild form of traumatic brain injury (TBI). Abnormal changes in cerebral function are thought to be responsible for the cognitive impairment following concussion acutely and possibly longer term. Specific markers of neurometabolic derangement have been reported during post-concussion in adults, but their manifestations are unknown in children with concussion. 1 In this study we applied in vivo 31P MRS technique on 7T human scanner to quantitatively assess an array of metabolic parameters, including concentrations of major phosphorous compounds, oxidized and reduced nicotinamide adenine dinucleotide (NAD+ and NADH), 2-3 intracellular pH and NAD redox ratio, in pediatric patients suffered from concussion. We detected cerebral metabolic changes in the patient at sub-acute phase as compared to age-matched controls, as well as the changes during subsequent recovery period after the head injury.METHODS
Study Participants: Ten pediatric concussion patients (11-17 years old, 9 males and 1 female) were recruited from the Pediatric Concussion Program or Emergency Department at Children’s MN with Glasgow Coma Scale (GCS) =13-15, post traumatic amnesia <24 hours, loss of consciousness <1 minute, and normal head CT/MRI. Their symptoms were evaluated using Post-Concussion Symptom Inventory for Parents (PCSI-P). 4 Each patient was scanned shortly after the initial injury and 4-6 weeks post injury. Six age-matched children were also recruited as neurologically normal controls (12-15 years old, 4 males and 2 females) for comparison.In vivo 31P MRS measurement and data analysis: The in vivo 31P MRS study was conducted on a 7T/90cm human scanner (Siemens MAGNETOM) with a 1H/31P (Dia.≈ 5cm) surface coil probe placed over the occipital lobe. After anatomic imaging and B0 shim, 31P spectra were acquired with following parameters: 300µs hard pulse for excitation, TR=3s and NT=320. For absolute quantification, 3D-CSI (FOV=12×12×9cm3, matrix=7×7×5, TR=1.2s, total NT=896) was acquired on each brain and an ATP phantom. 5 AMARES algorithm 6 in jMRUI software and a homemade Matlab program 2 were used for analyzing the 31P MRS spectra, and metabolite concentrations were determined using ATP as an internal standard. 5
RESULTS
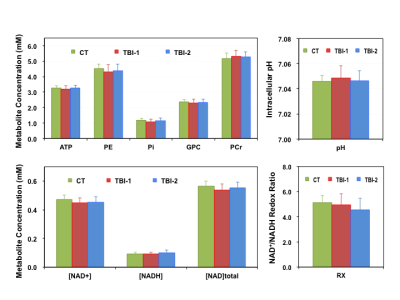
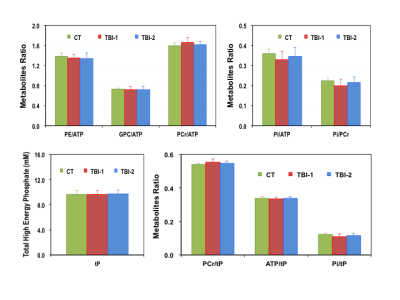
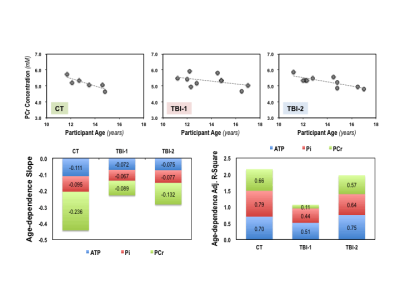
The intervals between initial injury and the time of MR scans are 6.8±1.5 days (TBI-1) and 44.0±7.1 days (TBI-2) for the concussion patients. Their PCSI-P scores are 3.3±4.5 (Pre-TBI), 17.7±16.6 (TBI-1) and 8.6±13.6 (TBI-2), respectively, and the mean recovery time determined based on their symptoms is ~23 days. Figure 1 summarizes the key metabolic parameters, i.e., the concentrations of ATP, PCr, Pi, phosphoethanolamine (PE), glycerophosphocholine (GPC), NAD+, NADH and total NAD ([NAD]total), intracellular pH and NAD redox ratio (RX=[NAD+]/[NADH) measured in the brains of controls (CT) and patients at two post-injury time points. It is clearly evident that the [ATP], [[Pi], [PE], [NAD+], [NAD]total and RX decreased, while the [PCr] and intracellular pH increased shortly after the injury. Except [NADH] and RX, most of the changes were partially reversed at the second scan, at least one month later. The ratios of various metabolites were also calculated from their concentrations and are summarized in Figure 2, where tP represents the total pool size of the free high-energy phosphates in the brain (tP=ATP+PCr+Pi). Interestingly, we found strong age-dependences (p<0.05) in several phosphorous metabolites, examples of [PCr] are shown in Figure 3; such relationships were significantly weakened after the head injury, which only partially recovered at the time of second scan.DISCUSSION and CONCLUSION
Little is known about the neurometabolic derangement in children with concussion. In this pilot study, we investigated the metabolic alterations in pediatric concussion brains using 31P MRS at 7T. We observed PCr/ATP and pH increases in patients shortly after mild concussion; this trend is consistent with what has been observed in adult patients after severe TBI. 7 In addition, we quantified absolute concentrations of seven phosphorus metabolites as well as intracellular pH and NAD redox ratio, which is more challenging than determining the ratio of high-energy phosphates commonly reported in literature. The detected metabolic changes between CT, TBI-1 and/or TBI-2 are relatively small (<10%), but they provide critical and previously unknown information for understanding the underlying severity of the injury and the degree of recovery in the developing brain. So far, there has been no report of decreased cerebral NAD+ and total NAD contents following head injury in pediatric patient, although this is not surprising, as initial injury likely activates the poly-ADP-ribose polymerase that consumes NAD+ as substrate. This notion is further supported by the slow NAD+ recovery observed in this study. We also detected strong age-dependences in high-energy phosphates and NAD contents in normal developing brains. Such relationships diminished after the injury, which were only partially restored after the concussion symptoms cleared. This intriguing finding confirms that the metabolic disturbance indeed plays an important role in the neuropathology of the concussion injury, especially in pediatric brains. It also suggests a possible cerebral vulnerability that exists beyond when concussion patients are determined clinically recovered from injury.In conclusion, the in vivo 31P MRS technique at ultrahigh field can detect metabolic and energetic changes in healthy and mild-TBI human brains with superior sensitivity; thus, it offers a unique tool and valuable metabolic markers for concussion research.
Acknowledgements
Education and Research Committee’s Internal Research Grant of Children’s Hospital & Clinic MN;
NIH Grants: R01 MH111413, R01 CA240953, R24 MH106049, U01EB026978, P41 EB027061, P30 NS076408.
References
- 1. McCrea M and Manley G. State of the Science on Pediatric Mild Traumatic Brain Injury: Progress Toward Clinical Translation. JAMA Pediatr. 2018;172(11):e182846. doi: 10.1001.
- 2. Lu M, Zhu XH, Zhang Y and Chen W. Intracellular redox state revealed by in vivo 31P MRS measurement of NAD+ and NADH contents in brains. Magn Reson Med. 2014;71(6):1959-72.
- 3. Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2876-81.
- 4. Sady MD et. al, Psychometric characteristics of the Post Concussion Symptom Inventory in children and adolescents. Arch of Clin Neuropsychology. 2014 April: 1-16
- 5. Zhu XH, Lee BY and Chen W. Functional energetic responses and individual variance of the human brain revealed by quantitative imaging of adenosine triphosphate production rates. J Cereb Blood Flow Metab. 2018;38(6):959-972.
- 6. Vanhamme L, van den Boogaart A and Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35-43.
- 7. Stovell MG et. al, Phosphorus spectroscopy in acute TBI demonstrates metabolic changes that relate to outcome in the presence of normal structural MRI. J Cereb Blood Flow Metab. 2018 Sep 18:271678X18799176. doi: 10.1177.
Figures