0917
Subcortical abnormality reveal disease specific changes in amyotrophic lateral sclerosis1Brain and Mind Centre, The University of Sydney, Sydney, Australia, 2Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, United Kingdom
Synopsis
Amyorophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative condition affecting the motor system, but increasingly recognised as a multi-system disease. We present two studies that highlight disease specific patterns of abnormality in the thalamus and corpus callosum that suggest regional variation in neural relay structures may be promising markers of disease progression in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease of the motor system, with a clinical, pathological, and genetic overlap with frontotemporal dementia 1. The neural signature of ALS is characterised by structural, functional, and metabolic dysfunction of the motor cortices and corticospinal tract 2. In recent years it has become increasingly evident that ALS pathology is not limited to the motor system and has a wide cortical reach with disease progression 3. Cortical dysfunction, however, does not occur in isolation in neurodgenerative conditions. We present two studies where the overall objective was to examine the regional integrity of two key subcortical neural relay structures (thalamus, corpus callosum) as a holistic marker of widespread cortical dysfunction, and their association with clinical features in ALS.Methods
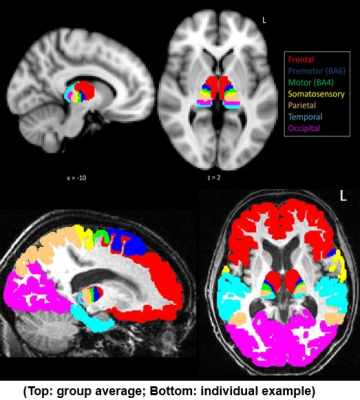
Clinically well-defined sporadic ALS patients and age-and-education matched healthy control participants (p values > 0.1) were recruited from the Oxford MND clinic, in accordance with ethical approval. All patients underwent comprehensive clinical examination and 3T structural and diffusion weighted MRI scans (Siemens Trio) on the same day.Study 1 (Thalamus): 20 ALS and 31 control participants were recruited with a subset of patients (n=11) scanned longitudinally after a 6 month delay. Overall grey matter volume and morphological change was assessed using FSL-FIRST. Structural T1 scans were processed in Freesurfer 6.0 to generate cortical masks of the frontal, premotor, motor, somatosensory, parietal, temporal, and occipital cortices. Masks were registered to native diffusion space using linear and non-linear transformation in FSL. Diffusion-based MRI tractography was carried out using PROBTRACKX to parcellate the thalamus based on structural connectivity to cortical masks. Diffusivity of thalamic parcellations were compared between patients and controls at baseline, and longitudinally between patients.
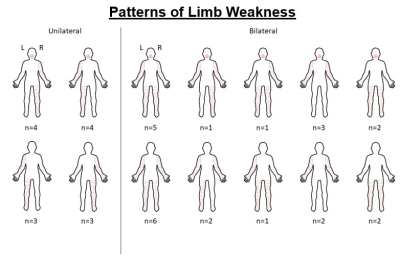
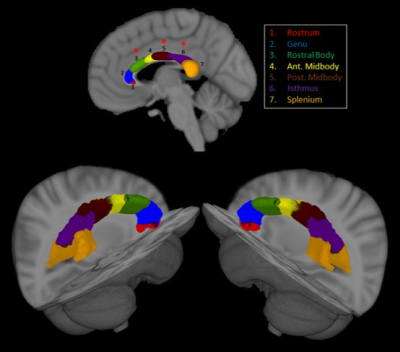
Study 2 (Corpus Callosum): 39 ALS and 25 control participants were recruited. Patients were further phenotyped into two groups based on their pattern of limb weakness at diagnosis (unilateral: n = 14; bilateral: n = 25; Fig. 1). Diffusion weighted MRI scans were preprocessed using MRtrix and probabilistic segmentation of the corpus callosum into 7 anatomical sections (rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus, splenium) was carried out using a neural network approach with TractSeg 4. Diffusivity of corpus callosum parcellations were compared between patients and controls.
Results
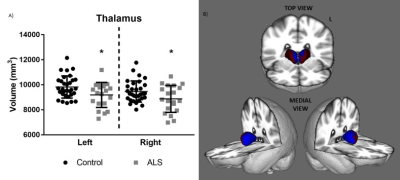
Study 1 (Thalamus): At baseline, ALS showed bilateral reduced thalamic volume (p < 0.03) and significant morphological change involving the medial surface of the thalami (p < 0.01) (Fig.2). The thalamus was consistently parcellated based on structural connectivity to the frontal, premotor, motor, somatosensory, parietal, temporal, and occipital cortices (Fig. 3). Patients showed significantly increased diffusivity (MD, RD, AD) in the left frontal, right somatosensory, and bilateral premotor, motor, and parietal thalamic parcellations compared to controls (p values < 0.05). Longitudinally, patients showed a significant increase in diffusivity (MD, RD, AD) in the right frontal lobe, and bilateral temporal lobe thalamic parcellations (p values < 0.05). Longitudinal change in diffusivity of the right frontal lobe thalamic parcellation showed a positive association with rate of functional decline in patients (r = 0.59; p = 0.05).Study 2 (Corpus Callosum): The corpus callosum was consistently parcellated into 7 segments (Fig. 4). Patients showed significant abnormal diffusivity in the rostral body (MD, AD), posterior midbody (FA, MD, RD), and isthmus (FA) compared to controls (p values < 0.04). Comparisons of patients based on limb weakness demonstrated those with bilateral limb weakness showed selectively reduced FA in the posterior midbody of the corpus callosum (p = 0.04). In contrast, patients with unilateral limb weakness showed widespread diffusivity changes throughout all segments of the CC excluding the rostrum (p values < 0.05).
Discussion & Conclusions
Together, the findings of regional abnormality in the integrity of the thalamus 5 and corpus callosum in ALS highlight a disease specific pattern of degradation that may reflect widespread cortical dysfunction. Both structures are important secondary sites of pathology with important associations to clinical features, namely the rate of functional decline and interhemispheric spread of limb weakness. Progressive subcortical abnormality, in particular regional variations within key neural relay structures, may be meaningful markers for monitoring in-vivo disease progression in ALS.Acknowledgements
The authors thank all study participants for their efforts and enthusiasm.References
1. Kiernan, M.C., Vucic, S., Cheah, B.C., et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942-955.
2. Turner, M.R., Agosta, F., Bede, P., et al. Neuroimaging in amyotrophic lateral sclerosis. Biomark Med. 2012;3:319-337.
3. Braak, H., Brettschneider, J., Ludolph, A.C., et al. Amyotrophic lateral sclerosis - a model of corticofugal axonal spread. Nat Rev Neurol. 2013;9:708-714.
4. Wasserthal, J., Neher, P., Maier-Hein, K.H. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage. 2018;183:239-253.
5. Tu, S., Menke, R.A.L., Talbot, K., et al. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2018;89:1250-1258.
Figures