0912
Neurochemical alterations in the visual cortex of glaucoma patients1Department of Ophthalmology, New York University, New York, NY, United States, 2Sackler Institute of Graduate Biomedical Sciences, New York University, New York, NY, United States, 3Department of Radiology, New York University, New York, NY, United States
Synopsis
Glaucoma is considered to involve neurochemical alterations in the visual system. While the role of excitotoxicity in glaucoma remains controversial, we showed that the balance between glutamate, a main excitatory signal, and gamma-aminobutyric acid (GABA), a main inhibitory signal, is involved in glaucoma pathogenesis. We demonstrated that the visual cortex of glaucoma patients changes to an excitatory-dominant state and that this change is driven by reduced GABA. Additionally, we showed that visual field loss is associated with reduced N-acetyl-aspartate, a marker for neuronal integrity. Taken together, these findings suggest that neurochemical alterations may serve as informative markers for glaucoma.
Introduction
Glaucoma is characterized by the progressive degeneration of the optic nerve and retinal ganglion cells. While its pathogenesis is still unclear, accumulating studies have shown that glaucoma involves alterations not only in the eye but also in the central nervous system1. In particular, excitotoxicity due to excessive amount of glutamate, a main excitatory neurotransmitter, has received increasing attention in recent decades2-4. Previous studies have demonstrated that injection of glutamate in the mammalian eye leads to severe degeneration of ganglion cells5. However, enhanced glutamate was not observed in a few studies involving humans and animals with glaucoma6,7. While glutamate remains to be controversial, a recent study proposed that inhibitory signals may be involved in glaucoma as well8. Based on these findings, we investigated alterations to the excitatory-inhibitory balance in the visual cortex in glaucoma. In addition, we examined how N-acetyl-aspartate (NAA), a marker for neuronal integrity, is affected by glaucoma.Methods
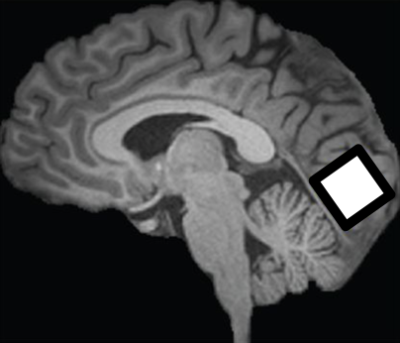
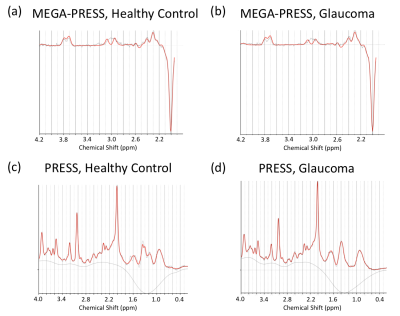
10 glaucoma patients and 4 healthy control subjects were scanned inside a 3-Tesla MRI scanner (Siemens MAGNETOM Prisma) with a 20-channel head coil at New York University Center for Biomedical Imaging. For anatomical localization, high-resolution T1-weighted MR images were acquired using a multi-echo magnetization-prepared rapid gradient echo sequence (256 slices, voxel size= 1×1×1 mm3). MRS for gamma-aminobutyric acid (GABA) was obtained using a MEGA-PRESS sequence (TR/TE= 1500/68 ms) with double-banded pulses. MRS for glutamate was obtained using a PRESS sequence (TR/TE= 3000/30 ms). The same single voxel (2.2×2.2×2.2 cm3) was placed along the calcarine sulci in the most posterior part of the occipital lobe bilaterally for both MEGA-PRESS and PRESS sequences (Figure 1). Glutamate and GABA were separately fitted by LCModel. We examined the reliability of quantification of each metabolite using Cramer-Rao Lower bounds (CRLB). We normalized the amount of GABA and glutamate using NAA values obtained from MEGA-PRESS, following LCModel guidelines9. We calculated the ratio between excitatory and inhibitory signals (E/I ratio) by dividing the amount of glutamate by that of GABA. Additionally, in order to examine whether our standard metabolite for MEGA-PRESS normalization in LCModel (NAA) was stable between groups, we normalized the amount of NAA with total creatine (tCr; combined creatine and phosphocreatine) for PRESS for further evaluation.Results
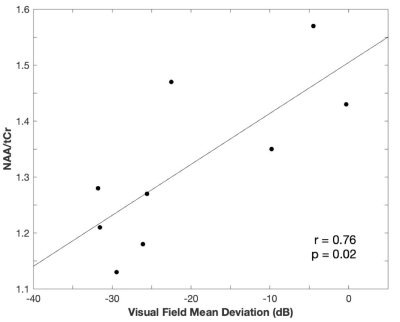
We confirmed the reliability of the quantification of GABA, glutamate, NAA, and tCr using CRLB. The mean (±s.e.m.) CRLB was 8.786±0.300% for GABA, 9.214±0.939% for glutamate, 2.00±0.000% for NAA, and 2.714±0.304% for tCr. The E/I ratio in the visual cortex was higher for the glaucoma group compared to the healthy control group (Figure 3a; average enhancement= 16.51%; t(12)= 1.897, P= 0.041, independent-samples t-test, 1-tailed). This difference was driven by GABA (Figure 3c). GABA was significantly lower for the glaucoma patients (Figure 3c; average reduction= 16.85%; t(12)= -3.105, P= 0.005, independent-samples t-test, 1-tailed), whereas glutamate was comparable between the two groups (Figure 3b; t(12)= -0.359, P= 0.363, independent-samples t-test, 1-tailed). In addition to glutamate and GABA, we further tested NAA’s stability in glaucoma, since this is the control metabolite used in MEGA-PRESS. Although we did not find a significant difference between groups, we observed a significant correlation between the amount of NAA and the clinical Humphrey 24-2 visual field mean deviation score (Figure 4; Pearson’s r= 0.746, P= 0.021).Discussion
Our study shows that the E/I ratio becomes enhanced in glaucoma and that this change is driven by GABA. GABA is known to play an important role in visual processing. For example, GABAergic interneurons are involved in sharpening feature selectivity in the primary visual cortex. Both optogenetic activation of GABAergic interneurons and administration of GABA were shown to increase visual feature selectivity10,11{Leventhal, 2003 #268}. These studies suggest that the visual function of glaucoma patients might have been impaired by a significant reduction of GABA. Furthermore, we did not find a significant change in glutamate. Although previous studies showed that excessive glutamate may be involved in glaucoma, recent studies did not observe enhanced glutamate6,7 and failed to ameliorate glaucoma using memantine, which targets glutamate excitotoxicity12. Therefore, more systematic studies are needed to understand the involvement of glutamate in glaucoma. In addition to glutamate and GABA, we found that greater visual field loss is associated with a larger reduction of NAA/tCr within glaucoma patients. It is noteworthy that GABA/NAA is reduced even if NAA is also decreasing, which further substantiates the reduction in GABA in the glaucomatous visual cortex. While NAA has been implicated in neuronal health in many neurodegenerative diseases13, tCr is presumably a stable metabolite. The changes in GABA and NAA metabolisms, if verified in larger studies, may potentially help guide more targeted interventions to glaucomatous neurodegeneration.Conclusion
Our results show that neurochemical alterations are involved in glaucoma pathogenesis. We demonstrate that the visual cortex adopts an excitatory-dominant state in glaucoma and that this imbalance is driven by GABA and not glutamate. Furthermore, we show that visual field loss is associated with a reduction in NAA within glaucoma patients. Taken together, these findings suggest that neurochemical alterations in MRS may serve as informative markers for glaucoma. Future directions include investigating the interactions between neurometabolites and the structure and function in the eyes and brains of glaucoma.Acknowledgements
This work was supported in part by the National Institutes of Health R01-EY028125 (Bethesda, Maryland); BrightFocus Foundation G2013077, G2016030 and G2019103 (Clarksburg, Maryland); Research to Prevent Blindness/Stavros Niarchos Foundation International Research Collaborators Award (New York, New York), and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (New York, New York).References
1. Murphy MC, Conner IP, Teng CY, Lawrence JD, Safiullah Z, Wang B, Bilonick RA, Kim SG, Wollstein G, Schuman JS, Chan KC. Retinal Structures and Visual Cortex Activity are Impaired Prior to Clinical Vision Loss in Glaucoma. Sci Rep-Uk. 2016;6. doi: ARTN 31464 10.1038/srep31464. PubMed PMID: WOS:000381188100001.
2. Faiq MA, Wollstein G, Schuman JS, Chan KC. Cholinergic nervous system and glaucoma: From basic science to clinical applications. Prog Retin Eye Res. 2019;72:100767. doi: 10.1016/j.preteyeres.2019.06.003. PubMed PMID: 31242454; PMCID: PMC6739176.
3. Lotery AJ. Glutamate excitotoxicity in glaucoma: truth or fiction? Eye (Lond). 2005;19(4):369-70. doi: 10.1038/sj.eye.6701623. PubMed PMID: 15806116.
4. Osborne NN, Chidlow G, Wood JP. Glutamate excitotoxicity in glaucoma: truth or fiction? By AJ Lotery. Eye (Lond). 2006;20(12):1392-4. doi: 10.1038/sj.eye.6702234. PubMed PMID: 16440018.
5. Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58(2):193-201. doi: 10.1001/archopht.1957.00940010205006. PubMed PMID: 13443577.
6. Carter-Dawson L, Crawford ML, Harwerth RS, Smith EL, 3rd, Feldman R, Shen FF, Mitchell CK, Whitetree A. Vitreal glutamate concentration in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci. 2002;43(8):2633-7. PubMed PMID: 12147596.
7. Honkanen RA, Baruah S, Zimmerman MB, Khanna CL, Weaver YK, Narkiewicz J, Waziri R, Gehrs KM, Weingeist TA, Boldt HC, Folk JC, Russell SR, Kwon YH. Vitreous amino acid concentrations in patients with glaucoma undergoing vitrectomy. Arch Ophthalmol. 2003;121(2):183-8. doi: 10.1001/archopht.121.2.183. PubMed PMID: 12583783.
8. Zhou X, Zhang T, Wu J. Brimonidine enhances inhibitory postsynaptic activity of OFF- and ON-type retinal ganglion cells in a Wistar rat chronic glaucoma model. Exp Eye Res. 2019;189:107833. doi: 10.1016/j.exer.2019.107833. PubMed PMID: 31618613.
9. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260-4. PubMed PMID: 11410943.
10. Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488(7411):379-83. doi: 10.1038/nature11312. PubMed PMID: 22878719; PMCID: PMC3422431.
11. Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812-5. doi: 10.1126/science.1082874. PubMed PMID: 12730605.
12. Khatib TZ, Martin KR. Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Curr Eye Res. 2019:1-12. doi: 10.1080/02713683.2019.1663385. PubMed PMID: 31475591.
13. Zhang Y, Chen X, Wen G, Wu G, Zhang X. Proton magnetic resonance spectroscopy ((1)H-MRS) reveals geniculocalcarine and striate area degeneration in primary glaucoma. PLoS One. 2013;8(8):e73197. doi: 10.1371/journal.pone.0073197. PubMed PMID: 24009739
Figures