0911
Why white matter matters – Interplay of white matter hyperintensities, white matter tracts, and processing speed – The Maastricht Study1Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 5School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands
Synopsis
White matter hyperintensities interfere with the course of white matter tracts, and disrupt connections between gray matter regions. This process might potentially underlie cognitive decline. In the large population-based Maastricht Study (n=5083), we found an association of lower processing speed scores with larger white matter hyperintensities and smaller total tract volumes in important processing speed related white matter tracts. These findings provide more insight into how white matter hyperintensities seem to influence the cognition-sensitive organization of white matter tracts.
Introduction
Cerebral small vessel disease (cSVD) is a potential cause of vascular cognitive impairment1. cSVD lesions, such as white matter hyperintensities (WMH), interfere with the courses of white matter tracts, and disrupt the connections between distributed gray matter regions2. These changes in white matter connectivity might potentially lead to general cognitive impairment, but also to decline in various specific cognitive domains, including memory, information processing speed and executive functioning3,4, which all rely on spatiotemporally orchestrated activation of large scale neuronal networks. According to the literature, processing speed is an overall measure of cognitive mechanisms that are involved in fluent execution of perceptual, cognitive, and psychomotor processes5. Therefore, processing speed is associated with the properties of connections between motor regions, and regions in the parietal, temporal, and occipital lobes6,7. Previous studies found associations of slower processing speed with smaller tract volumes in deep white matter tracts, such as the cingulum and the cortico-spinal tract, with smaller volumes8, and with larger WMH volumes9 of these specific tracts. However, the precise topology in which white matter connectivity is disrupted, how WMHs contribute, and how processing speed is affected remains unknown. Therefore, in this study, we aim to investigate the associations of the tract-specific total and WMH volumes with processing speed scores per tract.Methods
Subjects: Recruited in the Maastricht Study10, a population-based cohort study (n=5083, 21% type 2 diabetes (by design), 49% men, aged 40-75 years). The goal of The Maastricht Study is to increase insight into the causes and development of chronic disease, with the main focus on diabetes and cerebrovascular disease.MRI acquisition: Brain 3T MRI scans were acquired (MAGNETOM Prisma fit, Siemens Healthcare, Erlangen, Germany) by use of a 64-element head/neck coil. A 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR/TI/TE 2300/900/2.98 ms, 176 slices, 256x240 matrix size and 1.00 mm cubic voxel size) was acquired for anatomic reference. Diffusion-weighted MRI (dMRI) data were acquired using a diffusion sensitized echo-planar imaging (EPI) sequence (TR/TE 6100/57 ms, 65 slices, 100×100 matrix size, 2.00 mm pixel size, 2 mm transverse slice thickness, and 64 directions (b=1200 s/mm2) and three b=0 scans ).
Cognitive assessment: Cognitive performance scores were assessed with an extensive neuropsychological test battery with scores for the domains memory, processing speed, and executive attention.
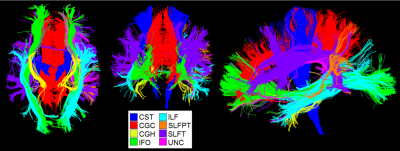
Image analysis: Structural scans were automatically segmented (with visual inspection) into different tissue types (e.g., white matter, and WMHs). First, we determined the spatial distribution of WMHs in this population. Subsequently, we determined the total and WMH volumes of sixteen important fiber tracts with automated atlas-guided tract reconstruction11 [Figure 1]. We transformed an atlas with 130 gray and white matter regions to diffusion-space12, and selected for each tract a specific subset of fibers which cross ‘AND’, and do not cross ‘NOT’ regions of interest (ROI) from the whole brain tractography11.
Statistical analysis: Multivariable linear regression analysis was used to investigate in which fiber tracts, tract-specific WMH volumes were associated with the cognitive scores, and if so, whether the volumes of these tracts also influenced the association with domain specific cognitive function. Associations were adjusted for age, sex, education level, log-transformed total WMH volume, intracranial volume (ICV), and cardiovascular risk factors (i.e., BMI, Total-to-HDL-cholesterol-ratio, systolic blood pressure, lipid-lowering-, and antihypertensive-medication, HbA1c, and prior cardiovascular disease).
Results
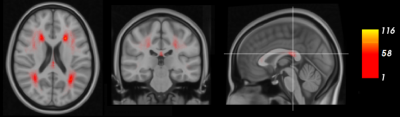
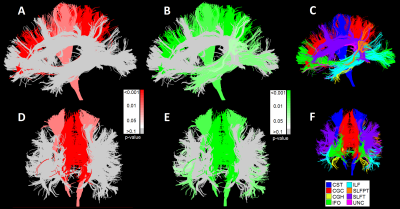
In Figure 2, the prevalence of WMHs in this population is visualized. Both periventricular WMHs, near to the lateral ventricles and located distant from the cerebral ventricle in the subcortical white matter, were found.Figure 3 depicts the statistical significance (p-values) of the associations between WMH (3A/D) and tract (3B/E) volumes with processing speed, independent of total WMH volumes and ICV. Lower processing speed scores were strongly associated with larger WMH volumes in the deeper located cingulum of cingulate gyrus and the cortico-spinal tract. Lower processing speed was also associated with smaller tract volumes of these two tracts, and the inferior-longitudinal fasciculus and the temporal part of the superior-longitudinal fasciculus. The association of lower processing speed with only tract volume in the ILF and SLFPT can be explained by the lower prevalence of WMHs in these regions. In contrast, for white matter tracts that were less pronounced to be associated with processing speed, such as the CGH, no associations of processing speed score with WMH or total tract volume were found.
Conclusion
In a large population-based imaging study, i.e., The Maastricht Study (n=5083), we observed that cognition, in terms of lower information processing speed, was associated with more extensive white matter hyperintensities and smaller total tract volumes, which reflects cognitive decrements due to white matter hyperintensities in individuals at mid-life age.Acknowledgements
No acknowledgement found.References
1. Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. The Lancet Neurology. 2002;1(7):426-36.
2. Langen CD, Cremers LGM, de Groot M, White T, Ikram MA, Niessen WJ, et al. Disconnection due to white matter hyperintensities is associated with lower cognitive scores. NeuroImage. 2018;183:745-56.
3. Dong C, Nabizadeh N, Caunca M, Cheung YK, Rundek T, Elkind MS, et al. Cognitive correlates of white matter lesion load and brain atrophy: the Northern Manhattan Study. Neurology. 2015;85(5):441-9.
4. van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39(10):2712-9.
5. Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological assessment: Oxford University Press, USA; 2004.
6. Usui N, Haji T, Maruyama M, Katsuyama N, Uchida S, Hozawa A, et al. Cortical areas related to performance of WAIS Digit Symbol Test: a functional imaging study. Neuroscience letters. 2009;463(1):1-5.
7. Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain : a journal of neurology. 2003;126(Pt 9):2093-107.
8. Magistro D, Takeuchi H, Nejad KK, Taki Y, Sekiguchi A, Nouchi R, et al. The Relationship between Processing Speed and Regional White Matter Volume in Healthy Young People. PloS one. 2015;10(9):e0136386-e.
9. Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Löffler M, et al. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. Journal of Cerebral Blood Flow & Metabolism. 2017;39(1):36-43.
10. Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439-51.
11. Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. NeuroImage. 2010;52(4):1289-301.
12. Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46(2):486-99.
Figures