0910
Modelling the temporal cascade of abnormalities in diffusion magnetic resonance imaging in sporadic Creutzfeldt-Jakob disease1Neuroradiology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy, 2Biomedical Imaging Group Rotterdam, Departments of Medical Informatics & Radiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands, 3National Prion Disease Pathology Surveillance Center, Case Western Reserve University, School of Medicine, Cleveland, OH, United States, 4National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, United States, 5Department of Pathology, Case Western Reserve University, School of Medicine, Cleveland, OH, United States
Synopsis
The subtypes of sporadic Creutzfeldt-Jakob disease (sCJD), determined only at autopsy, may have different abnormality patterns in diffusion-weighted magnetic resonance imaging (DW-MRI) according to few reports. For the first time, we provide temporal cascades of the DW-MRI abnormalities in seven distinct sCJD subtypes using a data-driven technique named “discriminative event-based model”. Based on these cascades, we propose a novel procedure to identify the subtype of a patient. We found that sCJD subtypes have either initial cortical (MM/MV1, MM/MV2C, VV1 subtypes) or subcortical involvement (MV2K and VV2) with specific orderings of DW-MRI abnormalities, allowing a correct subtype prediction in most cases.
INTRODUCTION
Sporadic Creutzfeldt-Jakob disease (sCJD) is the most common form of human prion diseases, which are rare and fatal neurodegenerative pathologies with a rapid course.1 sCJD has several subtypes (denoted as MM1, MV1, MM2C, MV2C, MV2K, VV1, and VV2) that differ in brain lesion distribution, clinical signs and survival times,2,3 and may respond differently to drug treatment.4To date, no diagnostic test can reliably distinguish sCJD subtypes ante mortem. MRI has sensitivity and specificity >95% for the early diagnosis of sCJD when diffusion-weighted imaging (DWI) is examined.5,6,7 It has been argued that characteristic DWI abnormality patterns may occur for each subtype,8 but these findings were not exploited for subtype diagnosis.
We aim to model DWI abnormality patterns in sCJD using a novel data-driven procedure and use this model to identify the subtype of patients in two scenarios: (i) using only MRI-derived data and (ii) including also the codon 129 genotype of the prion protein gene (PRNP129), a major determinant of disease variability.
METHODS
We collected the MRIs (DWI and ADC) of 453 sCJD patients with autopsy-confirmed subtype diagnosis. Brain MRIs were visually examined by one neuroradiologist blind to the diagnosis, grading the DWI signal hyperintensities on a 4-point ordinal scale, from “zero” (absence of CJD-related DWI hyperintensities) to “three” (extensive CJD-related DWI hyperintensities with low diffusivity on ADC maps). Twelve brain regions were scored: 5 cortical regions (frontal, temporal, occipital, parietal and separately precuneus), 3 limbic structures (cingulate, insula, hippocampus), caudate, putamen, thalamus and cerebellum.For each of the 7 sCJD subtypes, we applied the discriminative event-based model (DEBM),9 a novel data-driven technique that reconstructs the ordering of the 12 brain regions becoming abnormal in DWI.
First, each score was linearly converted into the probability of the corresponding region becoming abnormal. The subject-specific ordering of regions was established by sorting in a descending way the corresponding probabilities of a patient. The (central) ordering of each subtype was calculated as the ordering that minimized the sum of probabilistic Kendall’s Tau (pKT) distances to all subject-wise orderings of patients with the same subtype.
Second, we tested the model ability to predict the sCJD subtype of a patient as follows: for each subtype $$$i$$$, we obtained the likelihood $$$L_{i,j}=e^{-d(s_j,S_i)}$$$ from the pKT distances $$$d(s_j,S_i)$$$ between the subject-specific ordering of a patient ($$$s_j$$$) and the central ordering ($$$S_i$$$). The subtype with highest posterior probability $$$L_{i,j}\times p_i$$$ was assigned to patient $$$j$$$, where $$$p_i$$$ is the frequency of subtype $$$i$$$ in the sCJD population.
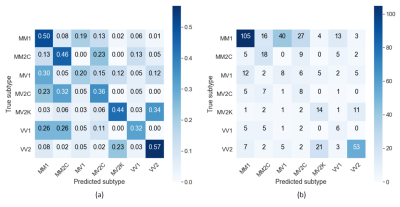
The balanced accuracy of the subtype prediction was assessed by leave-one-out cross-validation. Misclassifications between MM1 and MV1, or between MM2C and MV2C, were considered correct diagnoses, because they appear phenotypically indistinguishable.10 When the PRNP129 information (MM, MV or VV) was used, three separate classification problems were solved: MM1 vs MM2; MV1 vs MV2C vs MV2K; VV1 vs VV2.
RESULTS
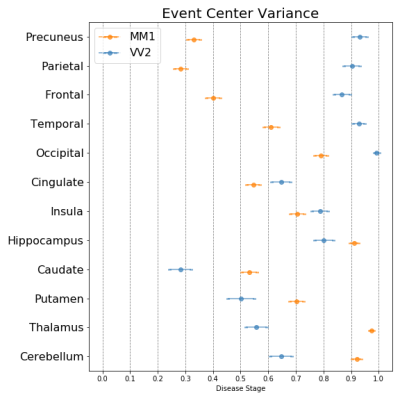
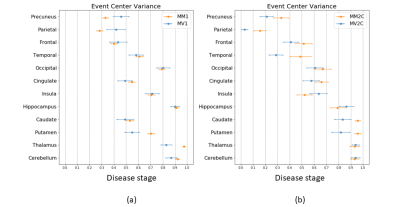
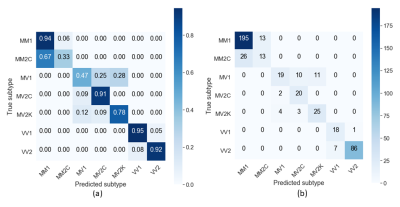
Figure 1 shows that the two most frequent sCJD subtypes (MM1 and VV2) had opposite orderings: initially, cortical regions were abnormal in MM1, followed by the striatum, and cerebellum and thalamus appeared at the end. Striatum was first in VV2 ordering, followed by thalamus and cerebellum, and cortical regions were the last. Misclassifications between these two subtypes were rare (only 3%, 10/301 MM1 and VV2 cases combined) (Table 1).As expected, MM1 and MV1 orderings were almost identical (Figure 2a), as well as those of MM2C and MV2C (Figure 2b). MM/MV2C and VV1 orderings were similar to that of MM1 (Figure 3a), with the main differences being the striatum affected earlier and the insula involved later in MM1. Most of the misclassified MM1 were assigned to MM/MV2C (21%,43/208) and VV1 (6%,13/208) (Table 1).
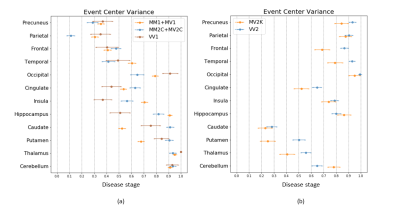
MV2K was similar to VV2, but with a later involvement of the cerebellum and an earlier occurrence of the frontal cortex (Figure 3b). These two subtypes were difficult to distinguish. On the contrary, MM/MV2C and VV1 were rarely confused with MV2K or VV2 (2.5%,2/80) and vice-versa (9.6%,12/125) (Table 1).
The balanced accuracy was 57% when using only MRI-derived data. The subtypes with highest percentages of correct diagnosis were MM/MV2C (82%,42/51) and MM/MV1 (67%,165/248) (Table 1). The balanced accuracy increased to 76% if the PRNP129 information was considered (MM: 64%, MV: 72%, and VV: 94%) (Table 2), and >90% of patients with MM1, MV2C, VV1 and VV2 subtypes were correctly identified.
DISCUSSION & CONCLUSION
We estimated the temporal cascades of DWI abnormalities in different sCJD subtypes and showed that subtype diagnosis is feasible with MRI. Two main groups of ordering were identified: (i) MM/MV1, MM/MV2C and VV1 showed an initial cortical involvement in the parietal region; (ii) VV2 and MV2K had an initial subcortical (striatum and thalamus) involvement, in keeping with recent neuropathological reports indicating that VV2 and MV2K share the same strain.10,11 Similar MRI progression patterns were previously found for MM1 and VV2,12,13 but our study is the first to use them for classification purposes.Our procedure reliably discriminated the sCJD subtypes between these two main groups, but lower classification accuracies were obtained within each group. This limitation was partially overcome by including the PRNP129 information, that allowed to distinguish MV2C from MV2K and VV1 from VV2 with accuracies >90%, but not MM1 from MM2.
Acknowledgements
RP, VV, EEB, SK and AB are supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 666992. PG is supported by NIH R01 NS083687 and P01 AI106705, and Charles S. Britton Fund. JB and BSA are supported by CDC NU38CK000480.
DISCLAIMER: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
References
- Parchi P, Strammiello R, Notari S, et al. Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: An updated classification. Acta Neuropathol. 2009;118(5):659-671.
- Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, Surewicz WK. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol. 2011;121(1):79-90.
- Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: Molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
- Appleby BS, Connor A, Wang H. Therapeutic strategies for prion disease: a practical perspective. Curr Opin Pharmacol. 2019;44:15-19.
- Young GS, Geschwind MD, Fischbein NJ, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: High sensitivity and specificity for diagnosis. Am J Neuroradiol. 2005;26(6):1551-1562.
- Vitali P, Maccagnano E, Caverzasi E, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76(20):1711-1719.
- Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
- Meissner B, Kallenberg K, Sanchez-Juan P, et al. MRI lesion profiles in sporadic Creutzfeldt-Jakob disease. Neurology. 2009;72(23):1994-2001.
- Venkatraghavan V, Bron EE, Niessen WJ, Klein S. Disease progression timeline estimation for Alzheimer's disease using discriminative event based modeling. NeuroImage. 2019;186:518-532.
- Parchi P, De Boni L, Saverioni D, et al. Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: An inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol. 2012;124(4):517-529.
- Bishop MT, Will RG, Manson JC. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc Natl Acad Sci U S A. 2010;107(26):12005-12010.
- Eisenmenger L, Porter M-C, Carswell CJ, et al. Evolution of diffusion-weighted magnetic resonance imaging signal abnormality in sporadic Creutzfeldt-Jakob disease, with histopathological correlation. JAMA Neurol. 2016;73:76-84.
- Baiardi S, Magherini A, Capellari S, et al. Towards an early clinical diagnosis of sporadic CJD VV2 (ataxic type). J Neurol Neurosurg Psychiatry. 2017;88(9):764-772.
Figures