0904
CEST imaging of self-healing hydrogels for drug delivery to the brain1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States
Synopsis
Self-healing hydrogels can adapt to the dynamic and mechanically demanding environment in the brain when compare to conventional brittle hydrogels. Herein, we developed a series of self-healing chitosan-dextran based hydrogels (CDgels) which were mechanically soft and its composition could be detected via CEST-MRI. Once crosslinked, CEST-MRI contrast at 1.2 ppm decreased when the crosslinking density increased. Interestingly, this phenomenon was observed when we further incorporated barbituric acid (BA) into CDgels to form part of the Schiff-base interaction. The resultant BA-loaded CDgels showed both CEST contrast at 3T, demonstrating a robust approach for imaging-guided hydrogel-based therapy in brain.
Introduction
Self-healing hydrogels are capable to recover to its designated hydrogel phase after injection. This unique property could avoid the potential risks of drug diffusion after transplantation [1-4]. Moreover, their self-healing nature makes them fit the irregular shape of the tumor resection cavity and provide intimate contact with the remaining cancer cells [5]. Currently, there is a lack of non-invasive methods for monitoring the extent of hydrogel gelation after injection in vivo. Chemical exchange saturation transfer (CEST) could be used for monitoring hydrogel compositions, degradation and the encapsulated cell viabilities detection [6-12]. Previous studies have shown dextran has abundant natural hydroxyl groups for CEST imaging [13, 14], and its oxidized form can readily form self-healing hydrogel with amine-based natural polymer through Schiff-base reaction, i.e. chitosan [15-17]. Herein, we developed a series of mechanically soft, self-healing and injectable CDgels with CEST detectability. The CEST contrast of hydrogel was decreased with crosslinking density and BA addition. Furthermore, both the CEST contrast of CDgels at 1.2 ppm and BA at 4.8 ppm could be monitored at 3T, showing potential in imaging-guided hydrogel-based drug delivery in the brain.Methods
Oxidized dextran (Odex) was prepared by addition NaIO4 (equivalent to theoretical oxidation degree of 5, 10, 15 and 20%) to dextran solution (2.5 g, 120 mL) [15-17]. The resulting mixture was stirred overnight at room temperature. Afterwards, an equimolar of diethylene glycol to NaIO4 was added to quench the reaction and then dialyzed for 3 days against distilled H2O and lyophilized to obtain pure Odex. CEC was synthesized by adding acrylic acid (1.88 mL) to chitosan solution (1 g, 50 mL) and stirred for 3 days at 50 oC [2]. The pH was then adjusted to 10-12 by NaOH solution. Afterwards, it was dialyzed for 3 days and lyophilized to get pure CEC. CDgels were prepared by mixing equal volume of Odex-6% (5.0 wt%) and various concentrations of CEC (1.0, 1.5 and 2.0 wt%) solutions under pH 6.5, and named CD-1, CD-1.5 and CD-2, respectively. The BA-loaded CDgels were prepared by firstly dissolving BA (1.0 wt%) in Odex-6% (5.0 wt%), and then mixed with CEC similarly. Rheological properties of the CDgels were tested by a rheometer (Kinexus pro+) with a parallel 20 mm plate at 25 oC. For the CEST imaging studies, all the Odex solution (2.5 wt%) and CDgels with or without BA were performed on a horizontal bore 3T Bruker BioSpec system (Bruker, Germany) using a 3s continuous-wave (CW) saturation pulse under 37 oC by a gas warming system, equipped with a 38 mm volume coil as transmitting and receiving. The B0 field was shimmed and a modified RARE sequence was used to generate the Z-spectrum. Images were acquired with the following parameters: B1=1.2 μT, Slice thickness=1 mm, field of view (FOV) =2016 mm, image size = 6464, RARE factor = 32, repetition time/echo time (TR/TE) = 5000/4.7 ms, -7 to 7 ppm or -5 to 5 ppm, 0.2 ppm steps.Results and discussion
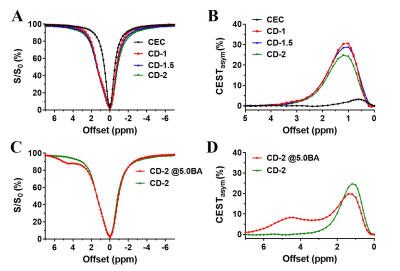
We prepared Odex with degrees of oxidation at 2%, 6%, 10% and 14%, which were determined by the hydroxylamine hydrochloride titration assay. As shown in Fig.1 A&B, dextran and its oxidized form showed CEST contrast at 1.2 ppm. The optimized power was 1.2 μT (Fig.1 C). The 6% oxidized Odex was selected for CDgels preparation because of the appropriate gelation time (35~200 s). After mixing, the storage modulus (G’) of CDgels increased over time (Fig. 2A) by the gradual formation of Schiff-bond [15-17]. All CDgels were mechanically soft with G’ in the range of 20-40 Pa at 10 Hz (Fig. 2B), which could deter the tumor cell proliferation and migration [19, 20]. As CEC concentration increased, the gelation time decreased, while the stiffness enhanced, due to the increase of available amine group and the resulting higher crosslinking density [15, 18]. Furthermore, they were injectable through a 23-G needle and exhibited superior self-healable property as the G’ rapidly recovered when switching the high oscillatory shear strain to low strain (Fig.2C, D&E). The CDgels also produced CEST contrast at 1.2 ppm same to Odex (Fig.3 A&B), but the contrast decreased with the increase of CEC concentration and stiffness, due to the slower diffusion rate at higher crosslinking hydrogel [21]. After incorporation of BA, both CEST contrast from CDgel (1.2 ppm) and BA (4.8 ppm) (Fig.3 C&D) could be detected simultaneously. Interestingly, a decrease at 1.2 ppm was observed upon BA addition, which could form part of the Schiff-based crosslinking network. This resembles the decrease at 1.2 ppm when the crosslinking density increased. This unique contrast properties of CDgels could facilitate imaging-guided therapy in the brain.Conclusions
Here, we developed self-healing and injectable CDgels with mechanical properties compatible to the brain. Our CDgels have CEST contrast at 1.2 ppm, which indicates the extent of gelation of the resultant hydrogel. Using this unique CEST contrast to monitor the crosslinking density of resultant hydrogels could facilitate hydrogel-based drug delivery to the brain.Acknowledgements
This study was supported by CityU: P9610362; P7200516; P6000660; P7004859; RGC: GRF-9042620; NSFC: 81871409-H1808.References
1. Wei Z, Yang JH, Liu ZQ, Xu F, Zhou JX, Zrínyi M, et al. Novel biocompatible polysaccharide‐based self‐healing hydrogel. Advanced Functional Materials. 2015; 25: 1352-9.
2. Qian C, Zhang T, Gravesande J, Baysah C, Song X, Xing J. Injectable and self-healing polysaccharide-based hydrogel for pH-responsive drug release. International journal of biological macromolecules. 2019; 123: 140-8.
3. Wei Z, Yang JH, Zhou J, Xu F, Zrínyi M, Dussault PH, et al. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chemical Society Reviews. 2014; 43: 8114-31.
4. Syrett JA, Becer CR, Haddleton DM. Self-healing and self-mendable polymers. Polymer Chemistry. 2010; 1: 978-87.
5. Huang W, Wang Y, Huang Z, Wang X, Chen L, Zhang Y, et al. On-demand dissolvable self-healing hydrogel based on carboxymethyl chitosan and cellulose nanocrystal for deep partial thickness burn wound healing. ACS applied materials & interfaces. 2018; 10: 41076-88.
6. Liang Y, Bar-Shir A, Song X, Gilad AA, Walczak P, Bulte JW. Label-free imaging of gelatin-containing hydrogel scaffolds. Biomaterials. 2015; 42: 144-50.
7. Shazeeb MS, Corazzini R, Konowicz PA, Fogle R, Bangari DS, Johnson J, et al. Assessment of in vivo degradation profiles of hyaluronic acid hydrogels using temporal evolution of chemical exchange saturation transfer (CEST) MRI. Biomaterials. 2018; 178: 326-38.
8. Dorsey SM, Haris M, Singh A, Witschey WRT, Rodell CB, Kogan F, et al. Visualization of Injectable Hydrogels Using Chemical Exchange Saturation Transfer MRI. ACS Biomaterials Science & Engineering. 2015; 1: 227-37.
9. Zhu W, Chu C, Kuddannaya S, Yuan Y, Walczak P, Singh A, et al. In Vivo Imaging of Composite Hydrogel Scaffold Degradation Using CEST MRI and Two‐Color NIR Imaging. Advanced Functional Materials. 2019: 1903753-62.
10. Lock LL, Li Y, Mao X, Chen H, Staedtke V, Bai R, et al. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano. 2017; 11: 797-805.
11. Jin T, Nicholls FJ, Crum WR, Ghuman H, Badylak SF, Modo M. Diamagnetic chemical exchange saturation transfer (diaCEST) affords magnetic resonance imaging of extracellular matrix hydrogel implantation in a rat model of stroke. Biomaterials. 2017; 113: 176-90.
12. Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater. 2013; 12: 268-75.
13. Liu G, Banerjee SR, Yang X, Yadav N, Lisok A, Jablonska A, et al. A dextran-based probe for the targeted magnetic resonance imaging of tumours expressing prostate-specific membrane antigen. Nat Biomed Eng. 2017; 1: 977-82.
14. Li Y, Qiao Y, Chen H, Bai R, Staedtke V, Han Z, et al. Characterization of tumor vascular permeability using natural dextrans and CEST MRI. Magnetic resonance in medicine. 2018; 79: 1001-9.
15. Weng L, Romanov A, Rooney J, Chen W. Non-cytotoxic, in situ gelable hydrogels composed of N-carboxyethyl chitosan and oxidized dextran. Biomaterials. 2008; 29: 3905-13.
16. Zhou L, Chen M, Guan Y, Zhang Y. Multiple responsive hydrogel films based on dynamic Schiff base linkages. Polymer Chemistry. 2014; 5: 7081-9.
17. Weng L, Chen X, Chen W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and N-carboxyethyl chitosan. Biomacromolecules. 2007; 8: 1109-15.
18. Jiang F, Tang Z, Zhang Y, Ju Y, Gao H, Sun N, et al. Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Biomater Sci. 2019; 7: 2335-47.
19. Ulrich TA, de Juan Pardo EM, Kumar S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Research. 2009; 69: 4167-74. 20. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005; 8: 241-54.
21. Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998; 31: 8382-95.
Figures