0902
In vivo MRI can assess differences in cell density and size of different Cryptococcus species in brain lesions.
Liesbeth Vanherp1, Kristof Govaerts1, Amy Hillen2, Jennifer Poelmans3, Katrien Lagrou4, Greetje Vande Velde1, and Uwe Himmelreich1
1Biomedical MRI, University of Leuven, Leuven, Belgium, 2Department of Cell and Molecular Biology, Karolinska Institutet, Stockholm, Sweden, 3Janssen Pharmaceutical Companies of Johnson & Johnson, Beerse, Belgium, 4Laboratory of Clinical Bacteriology and Mycology, University of Leuven, Leuven, Belgium
1Biomedical MRI, University of Leuven, Leuven, Belgium, 2Department of Cell and Molecular Biology, Karolinska Institutet, Stockholm, Sweden, 3Janssen Pharmaceutical Companies of Johnson & Johnson, Beerse, Belgium, 4Laboratory of Clinical Bacteriology and Mycology, University of Leuven, Leuven, Belgium
Synopsis
Multi-parametric MRI was correlated with in vivo Fibred Confocal Fluorescence Microscopy and histology in two preclinical models of cryptococcosis. Increased ADCs and T2 relaxation times were linked to differences in capsule size and associated fungal density in brain lesions caused by the two pathogenic fungi Cryptococcus neoformans and C. gattii. This provides not only a non-invasive means to assess one of the most important virulence factors of Cryptococci in preclinical research but may also affect patient management by providing a method for differential diagnosis.
INTRODUCTION
Cryptococcosis, which is caused by the opportunistic pathogens Cryptococcus neoformans and C. gattii, is the most common fungal brain infection [1]. Fungal infections of the central nervous system are associated with high mortality and morbidity. Infections mainly occur in immunocompromised patients, but can also affect immunocompetent people [2]. One of the most important virulence factors of cryptococci is their polysaccharide capsule, one of the most studied traits in these model organisms [3, 4]. Current methods to assess the capsule size are limited to ex vivo analysis, thereby precluding longitudinal analysis of changes in this dynamic structure. We hypothesize that differences in capsule size can affect the packing of cells inside a lesion and investigated the fungal cell density and size of cryptococcal brain lesions in living mice using intravital fibered confocal fluorescence microscopy (FCFM) and multi-parametric MRI. Hereby, the model strains C. neoformans H99 (CN) and C. gattii R265 (CG) were used to assess the differences in both species and potentially provide a basis for differential diagnosis.MATERIAL AND METHODS
Unilateral focal brain lesions were induced by stereotactic injection of 104 GFP-expressing cryptococci [5] (CG R265, n=6 and CN H99, n=5) in the striatum of female Balb/C mice. MR images were acquired on day 9 post infection using a 9.4/20 Biospec small animal MR system (20cm horizontal bore with actively shielded gradients of up to 600mT m-1, Bruker Biospin, Ettlingen, Germany) and a 7 cm linearly polarized resonator for transmission and an actively-decoupled mouse brain surface coil for receiving (Rapid Biomedical, Rimpar, Germany).The following MRI protocols were acquired:
- T2-weighted anatomical MRI (TR/ TE = 4200/ 36.2 ms, rare factor 8, slice thickness 0.5mm, in plane resolution 0.1mm
- Diffusion weighted MRI, b-values up to 1561 s mm-2, TR/ TE = 3000/ 27 ms
- T1-maps using RARE-VTR with TR=272, 350, 750, 1500 and 5000ms
- T2-maps using multi-slice-multi-echo MRI with 16 TE increments of 10ms, ranging from 10 to 160ms, TR = 2000ms
After sacrificing the animals, colony-forming unit (CFU) counting on brain homogenates was performed and the size of the fungal cells and their surrounding capsule was measured after india ink staining using a microscope with digital camera (Leica DM LS2 with ICC50W). Analysis was performed using the tool described by Dragotakes et al. [6].
RESULTS
Quantitative multi-parametric MRIMRI results are summarized in Figure 1. Lesions were characterized by higher apparent diffusion coefficients (ADC), T1 and T2 relaxation times than in the contralateral (normal) hemisphere. The ADC and T2 relaxation times were significantly higher in CG lesions than in CN lesions, without differences in the T1 relaxation time. Clear distinction between CG and CN infection was possible.
In vivo Fibred Confocal Fluorescence Microscopy
FCFM results are summarized in Figure 2. FCFM showed that the cryptococcal cell density was higher in lesions caused by CN H99 than by CG R265. The number of cells (CFU) per mm³ of lesion material was significantly higher in CN lesions, confirming more densely packed cells in CN lesions.
Ex vivo confirmation
India ink staining of the homogenized brain tissue showed that CG R265 cells are in general larger than CN H99 cells, with a thicker capsule around the cell and a larger cell body size, leading to a lower cell density compared to CN (Figure 3).
DISCUSSION
The higher ADC and T2 relaxation time in CG lesions compared to infections caused by CN can be explained by the larger fluid-filled intercellular spaces and higher amount of the highly hydrated capsular material in CG. This has been summarized in the model shown in Figure 4. The lower fungal density in CG R265 lesions was confirmed both by the FCFM images and by calculating the number of CFUs per lesion volume. India ink staining confirmed that CG cells were in general larger than CN cells, with a thicker capsule around the cell and larger cell body [3, 4], which explains the lower cell density compared to CN. While the larger capsule size for CG compared to CN confirms previous ex vivo studies [3, 4], more clinical strains are required to generalize our findings.We hereby propose a method that allows in vivo measurement of the fungal cell density in cryptococcal lesions, leading to indirect information about the capsule size in an in vivo setting. We are currently investigating the potential of FCFM and MRI to provide longitudinal insights in changes of fungal density throughout the disease progression.
CONCLUSIONS
Increased ADCs and T2 relaxation times were used to distinguish between CG R265 and CN H99 lesions, which was related to the capsule size and associated fungal density in the respective lesions. If confirmed by studies using more clinical strains this multi-parametric MRI approach could be used to differentiate between CN and CG. As manifestation and treatment of cryptococcosis caused by CN or CG is different [7], this may impact patient management in the future.Acknowledgements
We are thankful for financial support from the European Commission for the Infect-ERA project CryptoVIEW. LV is an SB PhD fellow at Research Foundation Flanders (FWO).References
- Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the Diagnosis and Treatment of Fungal Infections of the CNS. Lancet Neurol. 17: 362–372 (2018)
- Góralska K, Blaszkowska J, Dzikowiec M. Neuroinfections Caused by Fungi. Infection 46: 443–459 (2018)
- Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL. A Yeast under Cover: the Capsule of Cryptococcus neoformans. Eukaryotic Cell 2: 655–663 (2003)
- Zaragoza O. Basic principles of the virulence of Cryptococcus Virulence 10: 490–501 (2019)
- Voelz K, Johnston SA, Rutherford JC, May RC. Automated analysis of cryptococcal macrophage parasitism using GFP-tagged cryptococci. PLoS One 5:e15968 (2010).
- Dragotakes Q, Casadevall A. Automated Measurement of Cryptococcal Species Polysaccharide Capsule and Cell Body. J. Vis. Exp. e56957–e56957 (2018).
- Chen SCA, Meyer W, Sorrell TC. Cryptococcus gattii Infections Clin.
Microbiol. Rev. 27: 980-1024 (2014)
Figures
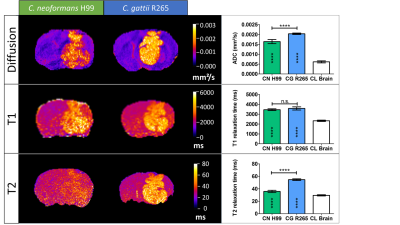
Quantification and representative images for CN H99 and CG R265 lesions. ADC maps, T1 maps and T2 maps are shown for a C. neoformans (H99) and C. gattii (R265) lesion.

Representative FCFM images and quantification of CFU counts and FCFM data for CN H99 and CG R265 lesions. Lesion volume (b) and cells/mm³ (d) were derived from MR images.

Quantification of India ink staining from brain homogenates for C. neoformans (H99) and C. gattii (R265).
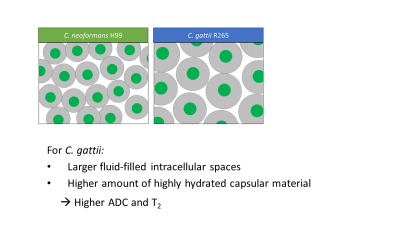
Proposed
model of differences between C. neoformans and
C. gattii in relation to the MRI contrast
properties