0895
Dual Flip-angle IR-FLASH for B1+ Insensitive T1 Mapping: Application to T1 CMR Multitasking.1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
T1 mapping is important for many diseases, from cancer [1] to cardiovascular disease [2], and more. Many fast T1 mapping protocols rely on an IR-FLASH sequence, especially in the heart. However, the accuracy and repeatability of T1 mapping with IR-FLASH are compromised by B1+ inhomogeneity. Here we present a simple dual-flip-angle (DFA) modification of the IR-FLASH pulse sequence to provide B1+-robust T1 mapping that obviates the need for a separate B1+ scan . We show the improved agreement of DFA-IR-FLASH to IR-TSE in a phantom study as well as its feasibility for in vivo cardiac T1 mapping with MR Multitasking.
Introduction
Quantitative mapping of longitudinal relaxation time T1 provides objective assessments of tissue state, with especially great potential for longitudinal and multi-center studies. The importance of T1 mapping has been identified in many diseases, from cancer [1] to cardiovascular disease [2], and more. Many fast T1 mapping protocols rely on variations of an inversion recovery fast low-angle shot (IR-FLASH) sequence [3], especially in the heart, where IR-FLASH with a short TR is suitable for additionally resolving cardiac and/or respiratory motion [4-8]. However, this sequence is subject to the Look–Locker effect, wherein the FLASH excitation pulses perturb T1 relaxation. Because the FLASH flip angle a depends on transmit efficiency B1+, the accuracy and repeatability of T1 mapping are compromised when the RF transmit field B1+ is non-uniform across the region of interest (ROI), in which case the Look–Locker effect is not properly corrected. Here we present a simple dual-flip-angle (DFA) modification of the IR-FLASH pulse sequence to provide Look–Locker- and B1+-robust T1 mapping that obviates the need for a separate scan to produce a B1+ map. We investigate the resulting T1 accuracy of DFA-IR-FLASH in a phantom study as well as its feasibility for in vivo non-ECG, free-breathing, cardiac T1 mapping within the MR Multitasking framework.Theory
The IR-FLASH signal equation produces an apparent T1, denoted as T1*:$$\frac{1}{T1^{*}}=\frac{1}{T1}-\frac{ln[\cos(B1^{+}\alpha)]}{TR} $$ (Eq.1).
Because this equation has two unknowns, T1 and B1+, there is an ambiguity that must be resolved in order to obtain T1 (Fig. 1a), either by assuming a B1+ value or by obtaining a B1+ map to pixelwise correct the value of a and thus of T1. Assuming a B1+ leads to inaccurate T1 in the presence of B1+ inhomogeneity or inefficiency; B1+ mapping typically requires at least one sequentially additional scan and is often sensitive to motion, which together amounts to time penalties and workflow complexity. An alternative approach to solving this ambiguity is to incorporate a second α or TR during acquisition, thereby creating two equations with two unknowns. In some contexts such as cardiovascular MRI, TR is often already selected to be very short to resolve motion, so extending TR could not only potentially lengthen scan time but also reduce temporal resolution. An more attractive solution may then be to vary α within the small tip angle regime (preserving proportionality with B1+), which does not affect timing. Here, we adopt this dual-flip-angle approach, alternating between 2 different flip angles in each inversion recovery period (Fig. 1b).
Methods
All data were acquired on a 3T Siemens Vida scanner. An in-house 14-vial T1 phantom was scanned, and 5 healthy subjects were consented and scanned in accordance with the Cedars-Sinai IRB protocol. 1-min Multitasking IR-FLASH scans were performed using either a 5 degree constant flip angle (CFA) with T1 fitting or alternating between 3 degree and 10 degree dual flip angles (DFA) with joint B1+ and T1 fitting. For reference T1 maps, IR turbo spin echo (IR-TSE) scans were performed on the phantom, and MOLLI T1 mapping scans [9] were performed on all 5 subjects. In the phantom, a reference B1+ map was obtained using TurboFLASH with RF preconditioning [10].Results & Discussion
Phantom scan results in Figure 2 demonstrate that DFA IR-FLASH has superior agreement to IR-TSE than CFA IR-FLASH. Figure 3 shows phantom T1 and B1+ maps. There is a smooth variation in B1+ in the reference B1+ map and the DFA IR-FLASH B1+ map, which induces T1 inhomogeneity in the CFA IR-FLASH T1 map, but not in IR-TSE or DFA IR-FLASH. In-vivo results in 5 subjects indicate the feasibility of the DFA IR-FLASH method. Figure 4 shows in vivo T1 and B1+ maps from one of the five subjects. The multitasking DFA B1+ map depicts smaller measures of the B1+ in the blood pool, reflecting the reduced Look-Locker effect occurring from blood inflow. This feature was present in scans of all 5 subjects. The CFA T1 map does not take inflow into account, and as a result overestimates T1 in the blood pool. Previous studies have had to use T1* to obtain blood pool T1 measurements [7], effectively assuming an effective B1+ of 0. Mean septal T1 values measured 1200ms for MOLLI, 1447ms for CFA multitasking, and 1317ms for DFA multitasking. MOLLI is known to underestimate myocardial T1 [11], so the apparent overestimation of CFA and DFA IR-FLASH with respect to MOLLI may represent improved accuracy in the myocardium, although this could not be confirmed in this study without comparison to a known accurate method such as SASHA [12].Conclusions
The addition of a second flip angle to IR-FLASH resolves T1–B1+ ambiguities. DFA IR-FLASH improved agreement to IR-TSE T1 estimates in vitro, and produced feasible T1 estimates of blood and myocardium in vivo. Joint B1+ and T1 fitting in DFA IR-FLASH appeared to absorb the effects of blood pool inflow into the B1+ map, as would be expected given the reduced effect of FLASH flip angles on the spin history of inflowing blood. An expanded study comparing accuracy and repeatability to a wider range of methods and in additional subjects is warranted.Acknowledgements
The authors extend their gratitude to Drs. Fei Han and Xiaoming Bi of Siemens A.G. for their advice on pulse sequence programming for this work. This work was supported by NIH R01 EB028146.References
[1] Lescher S, Jurcoane A, Veit A, Bähr O, Deichmann R, Hattingen E. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology. 2015 Jan;57(1):11-20.
[2] Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circ Res. 2016 Jul 8;119(2):277-99.
[3] Deichmann R, Haase A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson 1992 Feb;96(3):608-612.
[4] Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie Y, Wang N, Li D. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng. 2018 Apr;2(4):215-226.
[5] Weingärtner S, Shenoy C, Rieger B, Schad LR, Schulz-Menger J, Akçakaya M. Temporally resolved parametric assessment of Z-magnetization recovery (TOPAZ): Dynamic myocardial T1 mapping using a cine steady-state Look-Locker approach. Magn Reson Med. 2018 Apr;79(4):2087-2100.
[6] Becker KM, Schulz-Menger J, Schaeffter T, Kolbitsch C. Simultaneous high-resolution cardiac T1 mapping and cine imaging using model-based iterative image reconstruction. Magn Reson Med. 2019 Feb;81(2):1080-1091.
[7] Shaw JL, Yang Q, Zhou Z, Deng Z, Nguyen C, Li D, Christodoulou AG. Free-breathing, non-ECG, continuous myocardial T1 mapping with cardiovascular magnetic resonance multitasking. Magn Reson Med. 2019 Apr;81(4):2450-2463.
[8] Qi H, Jaubert O, Bustin A, Cruz G, Chen H, Botnar R, Prieto C. Free-running 3D whole heart myocardial T1 mapping with isotropic spatial resolution. Magn Reson Med. 2019 Oct;82(4):1331-1342.
[9] Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004 Jul;52(1):141-6.
[10] Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med. 2010 Aug;64(2):439-446.
[11] Teixeira T, Hafyane T, Stikov N, Akdeniz C, Greiser A, Friedrich MG. Comparison of different cardiovascular magnetic resonance sequences for native myocardial T1 mapping at 3T. J Cardiovasc Magn Reson. 2016 Oct 4;18(1):65.
[12] Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med. 2014 Jun;71(6):2082-95.
Figures
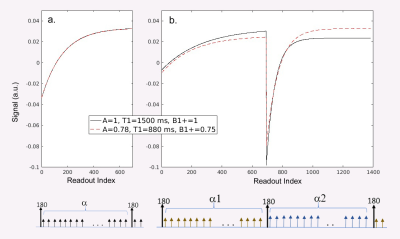
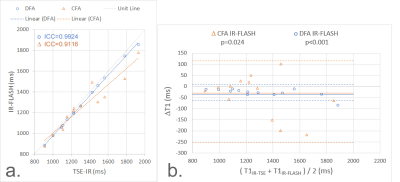
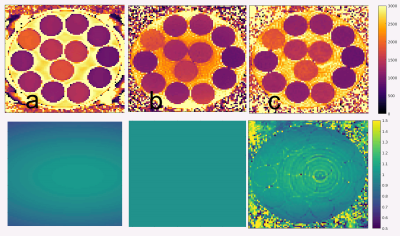
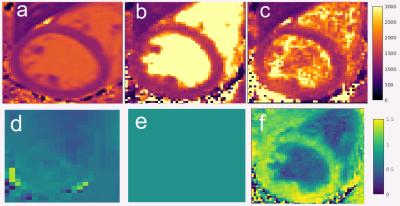
Figure 4. In-vivo cardiac T1 maps. Mean T1 values in the septum measured 1200 ms using (a) MOLLI; (b) 1447ms using CFA Multitasking IR-FLASH; and (c) 1317ms using DFA Multitasking IR_FLASH.The bottom row shows B1+ maps (d) reference, (e) constant, as assumed by CFA IR-FLASH, and (f) as fit from DFA IR-FLASH.