0892
Joint Calibrationless Multi-slice Multi-echo Parallel Imaging Reconstruction for Abdominal T2* Mapping1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Department of Electrical and Electronic Engineering, Southern University of Science and Technology, Shenzhen, China, 4School of Biomedical Engineering, Southern Medical University, Guangzhou, China
Synopsis
T2* mapping in abdominal imaging is challenging due to respiration motions that can result in severe artifacts and affect the accuracy of T2* quantification. Traditional simultaneous autocalibrating and k-space estimation (SAKE) provides a calibrationless parallel imaging approach to reduce the image acquisition time. However, SAKE does not utilize the highly sharable image contents and coil sensitivities among multi-slice multi-echo data. In this study, we proposed a joint calibrationless reconstruction of multi-slice multi-echo images from undersampled MR data for abdominal imaging. Results demonstrated that the resulting T2* maps were in excellent agreement with those from the fully sampled data.
Introduction
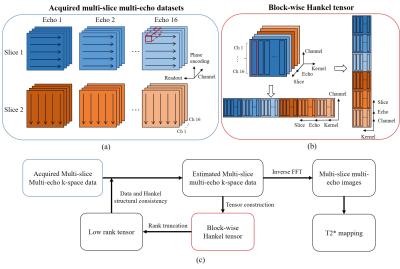
T2* mapping is a valuable technique in clinical diagnosis including liver iron and fat measurements. The most common method to map T2* is to acquire breath-hold multi-echo gradient-echo images. However, T2* mapping is challenging in the abdominal imaging since it is susceptible to respiratory and other physiological motions. Parallel imaging (PI) is a widely used technique for accelerated image acquisition by reducing phase-encoding steps through multiple receiving coils. However, PI reconstruction generally requires the coil sensitivity information from extra calibration scan1 or autocalibration signals2, thus reconstructed image quality is highly dependent on the accuracy of calibration data. For abdominal imaging, getting stable and accurate calibration data is difficult. Simultaneous autocalibrating and k-space estimation (SAKE)3 provides a solution to calibrationless PI. However, SAKE is based on single-contrast reconstruction. It is vulnerable to uniform undersampling, thus not highly robust in practice. More importantly, SAKE does not utilize the sharable image contents and coil sensitivities that are abundantly available among multi-slice multi-echo data. In this study, we proposed a multi-slice acquisition scheme where phase encoding direction orthogonally alternates among adjacent slices. We further presented a reconstruction method to jointly reconstruct undersampled multi-slice multi-echo k-space datasets through a block-wise Hankel tensor completion framework (ME-HTC). The proposed method produced excellent reconstruction results with high acceleration and without calibration data, yielding abdominal T2* maps that were comparable to those from the fully sampled data.Method
Data acquisitionFully sampled 16-channel 16-echo multi-slice abdominal datasets were acquired on a 3T Philips scanner with single breath-hold. The gradient echo was applied with TR = 18 ms, TEs = 1.376 to 17.027 ms with 1.043 ms increments, matrix size = 175×175, FOV = 350×350 mm2, flip angle = 25°, slice sickness/gap = 5/0 mm. The 1D undersampling masks were generated for retrospective undersampling along phase encoding direction in a uniform manner with acceleration factor R = 2, 3 and 4.
Image reconstruction
The proposed ME-HTC is formulated as a low-rank tensor completion problem as illustrated in Fig. 1. ME-HTC jointly reconstructs the multi-slice multi-echo in a calibration-free manner. In brief, it first acquires the undersampled k-space without calibration data by alternating phase encoding directions in adjacent slices. Then the multi-slice multi-echo k-space dataset are constructed into a block-wise Hankel matrix along kernel dimension and coil dimension respectively4. The sharable image contents and coil sensitivities among multi-slice multi-echo data are utilized in subsequently iterative low-rank tensor approximation and data consistency, to jointly reconstruct the multi-slice multi-echo abdominal images. For comparison, traditional SAKE method is also used to reconstruct individual multi-slice multi-echo images.
Results
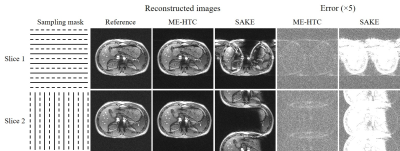
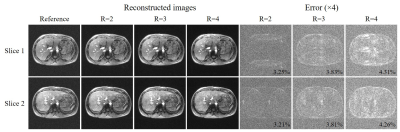
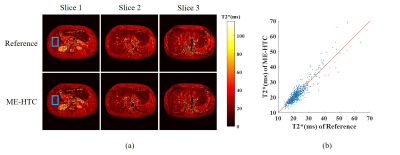
As shown in Fig. 2, traditional SAKE method could not cope with the uniform undersampling pattern without k-space central lines and exhibited severe aliasing artifacts. In contrast, the proposed ME-HTC could reconstruct two adjacent images jointly with virtually little artifacts. Fig. 3 shows that, with the increase of R, the ME-HTC multi-slice multi-echo images only suffered from slight artifacts. Fig. 4 demonstrates that the abdominal T2* values obtained from undersampled data using ME-HTC were in excellent agreement with those computed from images reconstructed by fully sampled data.Discussion and conclusions
This study demonstrated the multi-slice T2* mapping from multi-slice multi-echo images reconstructed using the proposed acquisition and ME-HTC joint reconstruction scheme without coil calibration. Here the acquisition involves alternating phase encoding directions between adjacent slices. The subsequent reconstruction scheme fully utilizes the abundant sharable information (coil sensitivities and image contents/supports) within the multi-slice multi-echo data. As a result, high-quality images can be reconstructed from the uniformly undersampled k-space data without coil sensitivity calibration, yielding superior performance while the traditional individual SAKE reconstruction completely failed. This new parallel imaging acquisition and reconstruction method leads to excellent T2* mapping results. In summary, our proposed acquisition and reconstruction method presents a new and calibrationless approach to accelerated abdominal T2* mapping.Acknowledgements
This study is supported in part by Hong Kong Research Grant Council (C7048-16G and HKU17115116 to E.X.W.), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer's Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
1. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952-962.
2. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002;47(6):1202-1210.
3. Shin PJ, Larson PE, Ohliger MA, Elad M, Pauly JM, Vigneron DB, Lustig M. Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion. Magn Reson Med 2014;72(4):959-970.
4. Yi Z, Liu Y, Zhao Y, Chen F, Wu EX. Joint Calibrationless Reconstruction of Highly Undersampled Multi-Contrast MR Datasets Using A Novel Low-Rank Completion Approach. ISMRM 2019, No.4746.
Figures