0890
T2-BUDA-gSlider: fast T2 mapping with blip-up/down acquisition, generalized SLIce Dithered Enhanced Resolution and subspace reconstruction1Center for Brain Imaging Science and Technology, Department of Biomedical Engineering, Zhejiang University, Hangzhou, China, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, charlestown, MA, United States, 4State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, China, 5Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Harvard-MIT Department of Health Sciences and Technology, Cambridge, MA, United States
Synopsis
We propose to combine the gSlider acquisition and blip-up/down acquisition (BUDA) to achieve high-resolution and distortion-free T2 mapping. Firstly, we incorporate Hankel structured low-rank constraint into BUDA reconstruction to recover distortion-free images from blip-up/down shots without navigation. To utilize the similarity among RF-encodings and TEs, we introduce a model-based shuffling-gSlider joint reconstruction to recover high-resolution thin-slice images by gradually eliminating the weak coefficient components during the iterative reconstruction. Finally, the reconstructed images are used to obtain quantitative T2 maps. The proposed method enables distortion-free high-quality whole-brain T2 mapping with 1 mm isotropic resolution within ~1 minute.
Introduction
High isotropic resolution T2 mapping has demonstrated potential in clinical and neuroscience applications1. 3D T2 mapping is generally encoding intensive and entails a prohibitively long acquisition2 while 2D encoding lacks the SNR to lend itself to high resolution imaging. Slice-dithered enhanced resolution (Slider)3 boosts the SNR-efficiency of 2D imaging using RF-encoded thick slice acquisition. Employing EPI readout in T2 mapping would further improve the acquisition efficiency, but come at the cost of geometric distortion. To mitigate these artifacts, two EPI acquisitions with inverted phase-encoding (with blip-up and -down) can be acquired to estimate the field map and perform distortion correction4.Herein, we combine the SNR-efficient gSlider with a rapid EPI readout for high-resolution T2 mapping. We perform joint reconstruction for two EPI shots using blip-up and -down acquisition (BUDA) with subspace modeling, and provide distortion-free, whole-brain T2 maps at 1 mm3 resolution within ~1 minute. Data/code:https://www.dropbox.com/s/lpi8j3eg353u9wq/t2_buda_gslider_lite.zip?dl=0.
Method
The proposed acquisition/reconstruction framework include the following steps:i. BUDA-gSlider acquisition. Figure 1A shows the sequence diagram of our simultaneous RF-encoded multi-slab BUDA 5x-gSlider acquisitions, where two interleaved blip-up/down shots are acquired for each of the five RF-encoding pulses sequentially. Five TEs: [49, 63, 73, 83, 103 ms] provide different T2 weighted contrast.
ii. BUDA reconstruction. With acquired interleaved blip-up and blip-down shots, distortion-free images are jointly reconstructed. Firstly, the blip-up and -down data were separately reconstructed using SENSE5 to obtain two individual images, which were imported into FSL TOPUP to estimate field maps. The estimated field maps were incorporated into low-rank constrained joint reconstruction6-8 for blip-up and -down data (Figure 1C), where Ft is the undersampled DFT in tth shot, Et is the off-resonance information, C are the ESPIRiT9 sensitivity maps, bt is the distortion-free image and dt is the k-space data. The constraint $$$\parallel H(b)\parallel_{*}$$$ enforces low-rank prior on the block-Hankel representation of the multi-shot data b, which is formed by concatenating the images bt from the two shots. Please note that the reconstructed images bt are RF-encoded slab images.
iii. Shuffling-gSlider joint reconstruction. In the initial step in Figure 2A, the number of acquired TEs was reduced from 5 to 3 for each RF-encoding and the missing data were synthesized using curve fitting. Both sampled and synthetic images were then combined to expand along the TE-RF dimension of and brought into the joint shuffling-gSlider model. By using the extended phase graph (EPG) algorithm10, a dictionary of T2 from 1 to 1000 ms was built. With principle component analysis, the first five components were selected as the temporal basis Φ11 shown in Figure 4B. This way, the desired high-resolution slab images could be expressed as , where is the temporal coefficient map. Figure 2C shows the shuffling-gSlider joint reconstruction:
$$min_{c_{exp}}\parallel A_{exp}\phi_{exp}c_{exp}-b_{exp}\parallel_2^2+\lambda_{Tik}\parallel c_{exp}\parallel_2^2$$
where $$$A_{exp}$$$ is the expanded RF-encoding matrix and $$$\parallel c_{exp}\parallel_2^2$$$ is the Tikhonov regularization. By utilizing the similarity of images with different TEs and RF encodings, temporal coefficient maps with high quality could be obtained.
iv. T2 estimation. T2 maps were generated by template matching the images with a pre-calculated dictionary.The data were collected using: Rin-plane=4, partial Fourier 6/8, multi-band factor=2, TR=2100 ms, 5 different TEs=49, 63, 73, 83, 103 ms (three out of five different TEs were acquired with the sampling pattern in Figure 2A), 26 thin slabs (thickness=5mm) with 5 RF encodings per slab (1-mm slice thickness for the final images), 1×1 mm2 in-plane resolution, FOV = 220×220×130 mm3.
Multi-TE single-echo spin-echo1 was implemented to provide gold standard T2 values using TR = 6000 ms and 7 TEs = 25, 50, 75, 100, 125, 150, 200 ms, resolution: 1×1×5 mm3.
Data were acquired on a 3T Siemens Prisma with a 64-channel head coil.
Results
Figure 3A shows the RF-encoded slab images of RF1/TE1 (i.e. 49 ms) using hybrid-SENSE12 (jointly reconstruct the blip-up/down shots with incorporated phase differences in the forward model but without low-rank constraint) and BUDA. BUDA results had higher SNR and reduced artifacts compared to hybrid-SENSE (red arrows). The individual SENSE reconstructions exhibit significant distortion compared to the 3D-FSE (blue arrows), while BUDA is consistent with the reference. Figure 3B shows the RF-encoded slab images of different TEs using BUDA, which provide the different T2 contrasts for subsequent T2 mapping.Figure 4 shows the T2 maps using straight-forward T2 fitting (direct gSlider reconstruction without shuffling) and proposed shuffling-gSlider joint reconstruction. T2 maps from the straight-forward T2 fitting approach are noisy. Compared with 5-TE acquisition (Figure 4B), the 3-TE acquisition (Figure 4C) shows similar quality of T2 maps but with faster acquisition (63 vs 105 sec).
Figure 5 shows two slices of T2 maps using the proposed method and the gold standard SE1. T2 values from four specific regions were evaluated and shown in the bar plots.
Discussion and Conclusion
The T2 values measured by the proposed method were in accordance with the gold standard SE, which confirmed its accuracy. The ability of BUDA to mitigate the distortion caused by off-resonance and eddy currents was validated by comparison against 3D-FSE. Incorporating partial Fourier and SMS into BUDA permitted whole-brain distortion-free T2 mapping with 1-mm3 resolution in about a minute. Limitations include the computation burden and the relatively narrow range of acquired TEs.Acknowledgements
No acknowledgement found.References
1. Singh P, Kaur R, Saggar K, Singh G, Kaur A. Qualitative and quantitative hippocampal MRI assessments in intractable epilepsy. Biomed Res Int. 2013;2013:480524.
2. Deoni SCL. Magnetic Resonance Relaxation and Quantitative Measurement in the Brain. In: Modo M, Bulte JWM, editors. Magnetic Resonance Neuroimaging: Methods and Protocols. Totowa, NJ: Humana Press; 2011. p 65-108.
3. Setsompop K, Fan Q, Stockmann J, Bilgic B, Huang S, Cauley SF, et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magn Reson Med. 2018;79(1):141-51.
4. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20(2), 870-888.
5. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magnetic Resonance in Medicine, 1999; 42(5), 952-962.
6. Low-Rank Modeling of Local k-Space Neighborhoods (LORAKS) for Constrained MRI. IEEE Transactions on Medical Imaging, 2014; 33(3): 668-681.
7. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS). Magn Reson Med. 2017;78(2):494-507.
8. Shin PJ, Larson PE, Ohliger MA, Elad M, Pauly JM, Vigneron DB, et al. Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion. Magn Reson Med. 2014;72(4):959-70.
9. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001.
10. Weigel M. Extended phase graphs: dephasing, RF pulses, and echoes - pure and simple. J Magn Reson Imaging 2015;41(2):266-295.
11. Tamir JI, Uecker M, Chen W, Lai P, Alley MT, Vasanawala SS, et al. T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging. Magn Reson Med. 2017;77(1):180-95.
12. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. Neuroimage. 2017;153:97-108.
Figures
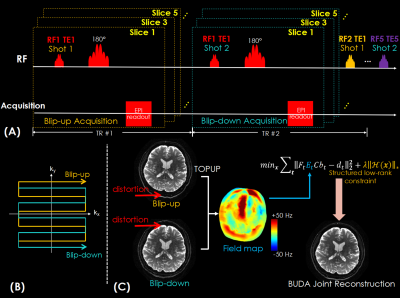
Figure 1.
(A) The sequence diagram of the BUDA-gSlider acquisition with multi-TE and spin-echo EPI readout.
(B) The acquisition trajectory of the blip-up/down 2-shots EPI.
(C) The flowchart of BUDA reconstruction. The individual reconstructed images by respectively using blip-up shot and blip-down shot are used to estimate the field map using FSL TOPUP. Then the field map is incorporated into the Hankel structured low-rank constrained forward model to jointly reconstruct distortion-free images.
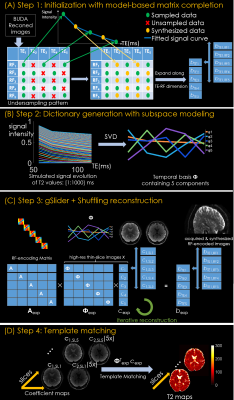
Figure 2. Reconstruction process including:
(A) Subsampling initialization step, where images of different TEs are subsampled and recovered by T2 fitting.
(B) Generating T2 dictionary by using EPG algorithm and corresponding temporal basis by principle component analysis.
(C) The gSlider-Shuffling joint reconstruction to generate the high-resolution thin-slice images by utilizing the similarity of images between different TEs and RF encodings.
(D) Template match the high-resolution thin-slice images with a pre-calculated T2 dictionary to obtain the final T2 maps.
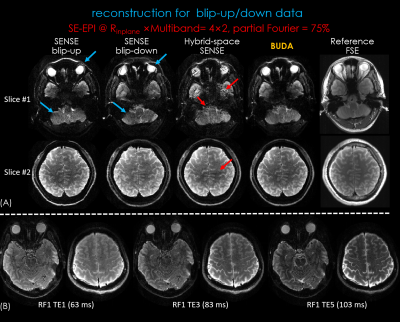
Figure 3.
(A) Reconstructed RF-encoded slab images of RF1/TE1 and reference 3D-FSE images. The images using BUDA reconstruction has reduced artifacts compared to hybrid-space SENSE and high geometric fidelity consistent to the reference images.
(B) RF-encoded slab images of different TEs by using BUDA reconstruction.
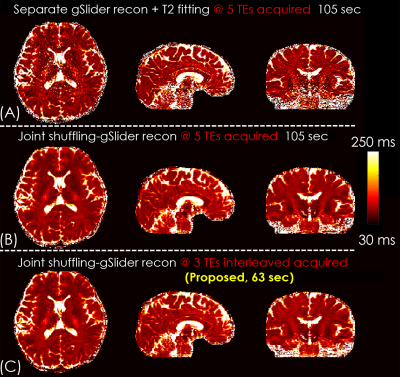
Figure 4.
The comparison of straight-forward approach (separate gSlider reconstruction and T2 fitting) and joint shuffling-gSlider reconstruction. With sub-sampled interleaved TEs acquisition, the scan time was reduced from 105 sec to 63 sec with similar image quality.
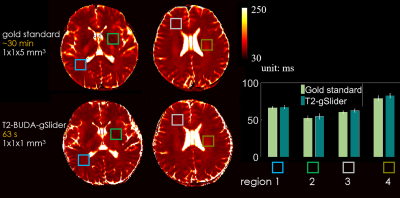
Figure 5.
The comparison between the proposed T2-BUDA-gSlider method and gold standard spin-echo method. The proposed method can achieve higher resolution (1×1×1 mm3 vs 1×1×5 mm3) and wider coverage (130 mm vs 55 mm in the slice direction) within short acquisition time (63 sec vs 30 min).