0884
Flexible model-based reconstruction through generalized cycled parameter splitting approach.1Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Model-based reconstruction approaches benefit from tight representation of the signal and from optimization on meaningful quantitative parameter maps, while requiring advanced algorithms and increased computational resources. We propose a generalized iterative thresholding algorithm with parameter splitting for model-based reconstruction, along with an efficient implementation. The approach is flexible and generalizable to problems in various MRI domains. We demonstrate it on the common image with phase evolution and signal decay model tackled with multi-echo GRE and Echo-Planar Time-resolved Imaging (EPTI), resulting in better image quality in comparison with GRAPPA and subspace constrained reconstruction, and increased z-scores in a low-SNR functional experiment.
Introduction
Model-based approaches[1-3] aim to reconstruct quantitative parameter maps directly from the signal, rather than obtaining them from reconstructed images, and thus benefit both from tight and MR-physics based constraining of the reconstruction problem, aiming for an accurate, less ill-defined problem and higher SNR. Model-based approaches also allow for a non-linear definition of the signal evolution, which may mitigate artifacts arising from linearization.We focus here on designing a general, flexible model-based reconstruction method based on iterative thresholding and parameter splitting, and applying it to gradient-echo and spin-echo imaging using a single chemical specie model[1].
Echo-Planar Time-Resolved imaging (EPTI) is an EPI variant with a zigzagging trajectory allowing for efficient coverage of the spatio-temporal signal evolution, providing distortion-free multiple-echo images along the acquisition time by accounting for the phase evolution due to field inhomogeneity and signal decay due to $$$T_2^*$$$. Similarly, a GRE-ME can be further accelerated by taking a PE/Echo CAIPI or per-echo Poisson sampling patterns. The spatiotemporal incoherence induced by such sampling patterns facilitates regularized joint reconstruction.
Theory
We model the image $$$I$$$ as a product of elements described as a composition of linear functions $$$L$$$ and nonlinear functions $$$s,u$$$ on parameter maps $$$x$$$, i.e. $$$\hat{I}=\Pi_i z_i$$$, $$$z_i=s_i(L_i(u_i(x_i))))$$$. Specifically, for multi-echo GRE and GE-EPTI, the signal model is $$I=me^{ip}e^{i2\pi b\odot TEs}e^{-TEs\oslash t},$$ with parameter maps $$$m,p,b,t$$$ representing the magnitude, phase, field inhomogeneity and $$$T_2^*$$$ and $$$\odot, \oslash$$$ being the elementwise multiplication and division. Although $$$p,b$$$ could be concatenated and treated in a joint fashion, we preferred to separate them, as it allows more flexibility in setting the constraints and the optimization parameters.Given the signal $$$y$$$, and the image-to-signal operator $$$A$$$, following[2] we suggest an iterative thresholding, parameter splitting approach, with the following update step:$$r=A^HA\hat{I}-A^Hy, q_i=\Pi_{j\neq i}z_j$$
$$x_{i,next}={prox}_{i,\alpha_i}(x_i+a_iu’(x_i)Re(L^H_iConj(s_i’(L_i(u_i(x_i)))q_ir)),$$
and cycling the update of the different parameter maps.
Further heuristics are added for specific parameter types. For image phase, phase-cycling is performed without the need for further manual specification, and the $$$T_2^*$$$ map is kept non-negative and within a reasonable range.
Methods
The method was implemented in BART[5], with GPU support and a linear-operator scripting language for a flexible, efficient description of $$$A$$$. The model was thus applied for Cartesian and non-Cartesian spiral EPTI (SCEPTI) trajectories.Realistic GRE-ME data was simulated in 230x320 matrix with 4-echoes and 6x subsampling or 10x undersampling Poisson pattern.
EPTI used FOV=24cm, matrix=120x120, 3-shots, R=4.
SCEPTI was based on 3ms out-in spiral, VD=1.3, target resolution 1.9mm, 48ms acquisition.
For comparison, we use the adaptation of the subspace constrained[6] reconstruction suggested for EPTI[7].
Results
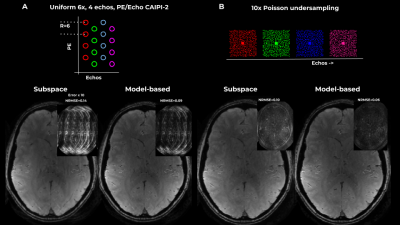
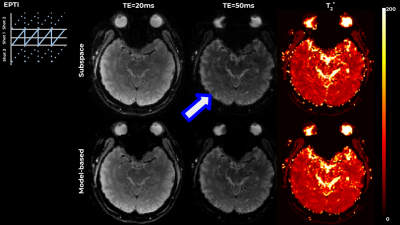
Figure 1 shows results on a simulated 4-echo GRE data, where the spatiotemporal space is covered by a PE/Echo CAIPI pattern or by per-echo Poisson sampling pattern. The suggested model-based approach outperformed subspace-constrained reconstrucion, and resulted in improved accuracy in terms of NRMSE and reduced artifacts in both cases.Figures 2 and 3 show results on in-vivo data acquired using 3-shot EPTI. The model-based approach outperformed subspace-constrained reconstruction, resulting in higher data-consistency and cleaner $$$T_2^*$$$ maps. Exponential decay requires several components for an accurate description, and the non-linear model allowed for a more constrained, yet well-defined problem. Additionally, the method optimized the T2* map directly, rather than infer it from reconstructed images.
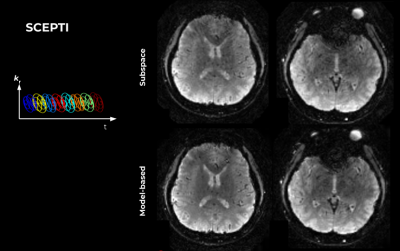
Figure 4 shows results on a 3-shot spiral EPTI (SCEPTI) acquisition. The combined challenge of undersampling under field inhomogeneities results in significant artifacts in the subspace-constrained reconstruction which are alleviated in the model-based approach.
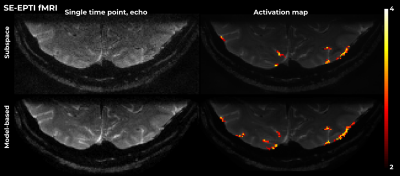
Figure 5 shows results for fMRI SE-EPTI. In this case, T2' was fixed in the model to be 500ms, as the SNR did not allow for the voxel-wise estimation of this parameter. A significant improvement in image SNR and artifact reduction is visible vis-à-vis subspace-constrained reconstruction. These benefits translate to higher z-scores in the functional analysis.
In all cases, reconstruction took ~10min per slice on a standard commercial GPU.
Discussion and Conclusion
A flexible model-based approach that allows for MR-physics based definition of the reconstruction problems, and for the parameter specific description of image/map manifold through proximal functions has been suggested, along with an efficient implementation. Application to anatomical and functional, Cartesian and non-Cartesian application has been shown.The system is easily translatable to a wide range of reconstruction problems in MRI.
Acknowledgements
This work was supported in part by NIH research grants: NIH R01EB019437, R01EB020613, R01 MH116173, and U01EB025162 .References
1. Sutton, Peltier, Fessler, Noll, Simultaneous estimation of I0, R2*, and field map using a multi-echo spiral acquisition, Proc. ISMRM 2002
2. Ong, F., Cheng, J. Y., & Lustig, M. (2018). General phase regularized reconstruction using phase cycling. Magnetic resonance in medicine, 80(1), 112-125.
3. Wang, X., Roeloffs, V., Klosowski, J., Tan, Z., Voit, D., Uecker, M., & Frahm, J. (2018). Model‐based T 1 mapping with sparsity constraints using single‐shot inversion‐recovery radial FLASH. Magnetic resonance in medicine, 79(2), 730-740.
4. Wang, F., Dong, Z., Reese, T. G., Bilgic, B., Katherine Manhard, M., Chen, J., ... & Setsompop, K. (2019). Echo planar time‐resolved imaging (EPTI). Magnetic resonance in medicine, 81(6), 3599-3615.
5. Uecker, M., Ong, F., Tamir, J. I., Bahri, D., Virtue, P., Cheng, J. Y., ... & Lustig, M. (2015, May). Berkeley advanced reconstruction toolbox. In Proc. Intl. Soc. Mag. Reson. Med (Vol. 23, p. 2486).
6. Liang Z. Liang, Z.P., 2007, April. Spatiotemporal imagingwith partially separable functions. In 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro (pp. 988-991). IEEE.
7. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-Resolved Imaging (EPTI) with subspace constraint and optimized kt trajectory. In: Proc Intl Soc Mag Reson Med. ; 2019.
Figures