0880
Prospective motion corrected 3D multi-parametric imaging
Naoyuki Takei1, David Shin2, Dan Rettmann3, Shohei Fujita4,5, Issei Fukunaga4, Akifumi Hagiwara4, Ken-Pin Hwang6, Marcel Warntjes7, Shigeki Aoki4, Suchandrima Banerjee2, and Hiroyuki Kabasawa1
1GE Healthcare, Tokyo, Japan, 2GE Healthcare, Menlo Park, CA, United States, 3GE Healthcare, Rochester, MN, United States, 4Juntendo University School of Medicine, Tokyo, Japan, 5The University of Tokyo Graduate School of Medicine, Tokyo, Japan, 6The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 7SyntheticMR, Linkoping, Sweden
1GE Healthcare, Tokyo, Japan, 2GE Healthcare, Menlo Park, CA, United States, 3GE Healthcare, Rochester, MN, United States, 4Juntendo University School of Medicine, Tokyo, Japan, 5The University of Tokyo Graduate School of Medicine, Tokyo, Japan, 6The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 7SyntheticMR, Linkoping, Sweden
Synopsis
To aim for reliable parametric mapping to motion artifact, prospective motion correction was integrated to a multi-parametric technique, 3D QALAS. 2D Spiral navigators were inserted into wait times in the QALAS without impacting scan time for motion tracking and correction. The effectiveness of prospective motion correction was demonstrated. The proposed technique is expected to yield prospectively motion corrected 3D brain volumetric images of multiple contrasts and quantitative mappings.
Introduction
Quantitative mapping is a promising tool for understanding the biophysical mechanisms related to diseases. Previously the clinical utility of MAGiC a method based on a single multi-delay multi-echo 2D fast spin echo scan and synthetic MRI data processing have been demonstrated for several clinical applications1,2. 3D QALAS 3,4(3D-quantification using an interleaved Look-Locker acquisition sequence with T2 preparation pulse) is a rapid quantitative mapping technique to provide 1mm3 iso-voxel high resolution volumetric images and 3D T1 and T2 , PD parameter maps from a 6-7 min single scan of the whole brain5 lending to multiplanar reformats and 3D parametric maps. However, to be clinically applicable, reliable quantitative measurement is crucial for reducing the incidence of motion artifact caused by uncooperative populations such as those with dementia or even by cooperative subjects with physiological motion such as respiratory-related movements and swallowing movement6. In this work, a real-time prospective motion correction (PROMO)7was integrated into a 3D acquisition based off QALAS and feasibility study was performed aiming for reliable parameter mapping.Method
Figure 1 shows schematic diagrams of our proposed method which is based off on 3D QALAS with 2D single-shot spiral navigator pulse sequences inserted into the dead times of the acquisition such that there is no scantime penalty. The three orthogonal navigators (SpNav) were played out just after 3D segmented data acquisition blocks 3, 4, and 5. The reconstructed images from three SpNavs were passed as input to the extended Kalman filter (EKF) algorithm for motion tracking and correction. Six dimensional motion parameters for translations and rotations in x-y-z space were calculated. The navigator and imaging sequences were updated in real-time for frequency, phase and slice encoding of the image volume for prospective rigid motion correction. Rescans were performed when large k-space inconsistency happens because transient motion can’t be captured between SpNavs.Our proposed method obtains five image contrasts using 3D gradient echo sequence in Fig.1. The first acquires MR data after T2 preparation pulse. The second and later readouts are performed during the following T1 relaxation after Inversion pulse. Five data collections are executed at equal time intervals of 1 second. The 3D data is acquired with segmented Cartesian k-space sampling. Quantitative T1, T2 and proton density maps were reconstructed from the obtained images using a research version of SyMRI (SyntheticMR, Linkoping, Sweden).
SpNav uses spiral acquisition in the following parameters: TR/TE=15.3/0.9 ms, FOV 32cm, 30 mm thickness, rBW 125kHz, reconstructed MAT 128x128. Twenty-four axial spiral images are acquired at the beginning of the scan and registered to a reference to determine the initial position of the navigators and the location of an anatomical mask applied to the navigators to localize motion detection to the brain.
To minimize the impact of signal saturation by SpNav on the image volume, flip angle of SpNav was optimized with cylindrical phantoms including white matter (T1: 588ms, T2: 79ms), gray matter(T1: 808ms, T2: 99ms) and CSF(T1:4061, T2:1639ms). Navigator slice thickness 10mm was used to show the saturation effect clearly. Flip angles of 3,6,10 and 30 degree were used.
To assess the effectiveness of motion correction, healthy volunteer scan was performed with motion correction on and off, where the subject was asked to repeat staged motions during scan. A 3.0 T System (Discovery MR 750w, GE Healthcare, Waukesha, WI, U.S.A.) with 12 channel coils (GEM HNU, GE Healthcare) was used. Other Imaging parameters were: spatial resolution 1x1x1.4mm3, TR/TE 6.5/2.2 ms, bandwidth 62.5kHz, flip angle 4 degree. Parallel imaging factor 2, scan time 4:55.
Results
Fig.2 showed the result of signal saturation effect by SpNav. The use of flip angle 3 deg showed no banding artifact on source image of Acquisition 4 in the QALAS and similar signal profile to flip angle 0 deg which means that no SpNav is used. Other #Acquisitions of the QALAS had the same result which is not shown here. Fig.3 displayed representative source images of the QALAS with motion correction on and off. Motion correction was effectively applied to reduce motion artifact. Dominant motion was translation x as the left-right direction and rotation z as the inferior-superior direction. Maximum translation and rotation were 1.94 mm and 3.3 degree, respectively. Fig.4 and Fig.5 were synthetic multi-contrast images and quantitative mappings generated with syMRI. Motion artifact from source image was propagated to the final synthetic images.Discussions and conclusion
Motion still poses one the biggest challenges to consistent MR image quality since some amount of head motion is typical in clinical subjects, and frequent in uncooperative cohorts. We successfully demonstrated the effectiveness of prospective motion correction in multi-parametric imaging such that signal intensity in source image was not impacted by SpNav acquisition. Scan time was maintained by inserting SpNav into the dead time of the QALAS except for rescan functionality, which is a reasonable trade-off to ensure more reliable measurement with small time penalty, instead of having to repeat the entire scan. The proposed prospectively motion corrected 3D multicontrast method yielding volumetric images of multiple contrasts such as T1, T2 , T2 FLAIR, PSIR and 3D parametric maps could significantly improve scan workflow, productivity and information/scantime and would be feasible in clinical settings.Acknowledgements
No acknowledgement found.References
- L.N. Tanenbaum, A.J. Tsiouris, A.N. Johnson, et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am. J. Neuroradiol., 38 (June (6)) (2017), pp. 1103-1110.2.
- A. Hagiwara, M. Hori, K. Yokoyama, M.Y. Takemura, C.Andica, T. Tabata, et al.Synthetic MRI in the detection of multiple sclerosis plaques. AJNR Am J Neuroradiol, 38 (2017), pp. 257-2633.
- Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. J Cardiovasc Magn Reson. 2014 Dec 20;16:102. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS.
- Hwang et al, Proc. Intl. Soc. Mag. Reson. Med. 18 (2019), 56275.
- Fujita S, Hagiwara A, Hori M, Warntjles M, Kamagata K, Fukunaga I, Goto M, Takuya H, Takasu K, Andica C, Maekawa T, Takemura MY, Irie R, Wada A, Suzuki M, Aoki S. 3D quantitative synthetic MRI-derived cortical thickness and subcortical brain volumes: scan-rescan repeatability and comparison with conventional T1-weighted images. J Magn Reson Imaging. 2019 ; 63:235-243.6.
- Watanabe K, Kakeda S, Igata N, Watanabe R, Narimatsu H, Nozaki A, et al. Utility of real-time prospective motion correction (PROMO) on 3D T1-weighted imaging in automated brain structure measurements. Sci Rep. 2016;6:38366.7.
- White N, Roddey C, Shankaranarayanan A, Han E, Rettmann D, Santos J, et al. PROMO: Real-Time Prospective Motion Correction in MRI Using Image-Based Tracking. Magnet Reson Med. 2010;63(1):91–105.
Figures
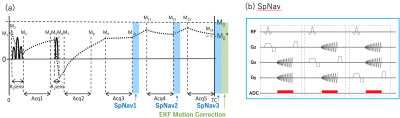
Fig.1. (a) Spiral navigator inserted 3D QALAS (3D-quantification using an interleaved Look-Locker acquisition sequence with T2 preparation pulse) is based on a 3D GRE using inversion recovery with interleaved T2 preparation. EKF motion correction is performed after three SpNavs. (b) Three orthogonal 2D spiral navigator (SpNav) for image based motion tracking.
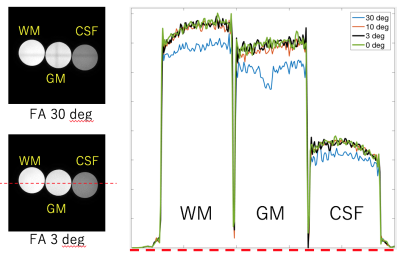
Fig.2. Signal saturation effect by SpNavon image volume. The left hand is slice image on Acquisition 4 in the QALAS with flip angle (FA) 30 deg and 3deg. The right hand is signal intensity profile of 30 deg, 10 deg, 3 deg and 0 deg along red dotted line on image of FA 3. FA 3deg is similar signal profile to that of 0 deg.
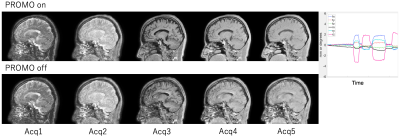
Fig.3. The effectiveness of prospective rigid motion correction. Representative raw slice image on image volume with the QALAS. The upper is PROMO on, the lower is PROMO off. Motion estimation during PROMO on was shown on the right figure.
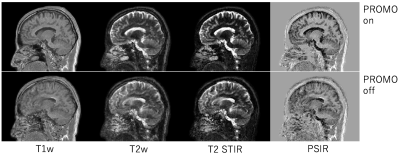
Fig.4. Synthetic multi-contrast images. The upper is PROMO on, the lower is PROMO off. Motion artifact is propagated into multi-contrast images from source images.
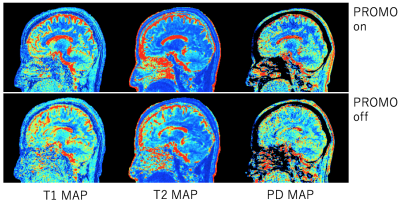
Fig.5. Parametric mappings. The upper is PROMO on, the lower is PROMO off. Prospectivemotion correction provides clean quantitative mapping during scan with motion.