0879
Free-Breathing Simultaneous Quantification of Liver T1, Fat and R2* with Variable Flip Angle Golden-Angle-Ordered 3D Stack-of-Radial MRI1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Physics and Biology in Medicine, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Quantification of T1, proton-density fat fraction (PDFF), and R2* in the liver can provide information about a range of diseases. Existing Cartesian acquisition schemes generally require breath-holding, which limits spatial coverage and may be difficult for sick, elderly or pediatric patients. In this study, we propose a variable-flip-angle (VFA) golden-angle-ordered (GA) 3D stack-of-radial sequence that can provide multiparametric mapping with volumetric liver coverage in three minutes during free-breathing and with intrinsic motion compensation capability. Pilot studies in healthy subjects demonstrate agreement with reference breath-held scans and good measurement repeatability.
Introduction
Quantitative MR parameters can characterize liver tissue status in several disease processes, such as T1 for fibrosis1‑3, proton-density fat fraction (PDFF) for steatosis4‑6 and R2* for iron overload7. Simultaneous multiparametric mapping in the liver can be achieved with variable-flip-angle (VFA) multi-echo Cartesian techniques8, but it requires multiple breath-holds with different flip angles, which limits spatial coverage and may be challenging for certain patients. VFA T1 calculations also require separate B1+ field maps for flip angle calibration, which may have errors due to potential image mismatch. Moreover, respiratory motion can cause biases in R2* quantification9. Golden-angle-ordered stack-of-radial MRI offers self-navigating capabilities to reconstruct images at a particular respiratory state for accurate VFA T1 and R2* quantification. Acquisition during free-breathing provides opportunities for larger spatial coverage. Here we propose a 3D multi-echo gradient-echo golden-angle-ordered radial VFA sequence10 that takes advantage of these properties to simultaneously quantify liver T1, PDFF, and R2* accurately and repeatably in a single 3-minute free-breathing scan.Methods
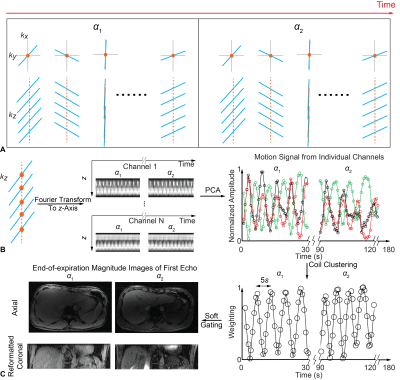
Imaging SequencesA 3D stack-of-radial trajectory with GA-ordered multi-echo readouts was used (Fig.1A) and repeated with two flip angles (“radial VFA”) for T1 quantification. Radial spokes with the same radial angle were collected along kz, and their (kx,ky)=0 points were Fourier-transformed to obtain projection profiles in z for all channels, which captured motion for both flip angles. Principal component analysis (PCA) was then performed to extract the first two principal components. Finally, channels exhibiting similar results (95% correlation threshold) were clustered to generate motion signal11,12 (Fig.1B). Radial data were assigned linear weights according to their motion signal amplitude, i.e. soft gating13, and was then re-gridded and channel-combined14 to produce complex images with the liver at end-expiration (Fig.1C). Magnitude images of all echoes were combined via sum-of-squares for VFA T1 fitting15. PDFF and R2* were calculated with the complex multi-echo images acquired with 3° flip angle to avoid T1-bias, using a seven-peak fat model16 and single effective R2* per voxel17.
Experiments
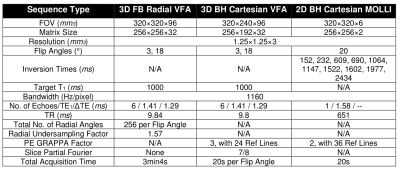
In an IRB-approved study, n=12 healthy adult subjects (6 males, 6 females) aged 31±10 years with body mass index of 23.2±4.1kg/m2 provided written informed consent and underwent two free-breathing radial VFA scans (acquired >10 minutes apart) to assess its repeatability. As references, one Cartesian gradient-echo VFA scan and one Cartesian modified Look-Locker inversion recovery (MOLLI)18 scan were acquired during breath-hold at end-expiration. To calibrate flip angles for VFA T1 fitting, B1+ maps were acquired during breath-holding at end-expiration19. All scans were conducted on a 3T scanner (Prisma, Siemens) with body and spine arrays. Table 1 lists scan parameters.
Data Analysis
In each subject, regions of interest (ROI) of 5cm2 were drawn in 3 slices (near liver dome, mid-section and lower-section) near corresponding landmarks in radial VFA and Cartesian VFA acquisitions to evaluate agreement in T1, PDFF and R2*. The repeatability of radial VFA T1, PDFF, and R2* was assessed using the intraclass correlation coefficient (ICC) in the same ROIs. 12 additional ROIs were drawn in 1 slice imaged by both radial VFA and Cartesian MOLLI acquisitions to evaluate T1 agreement.
Results
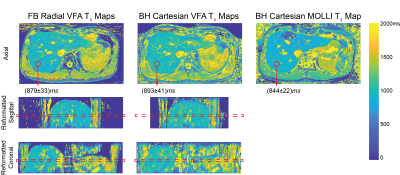
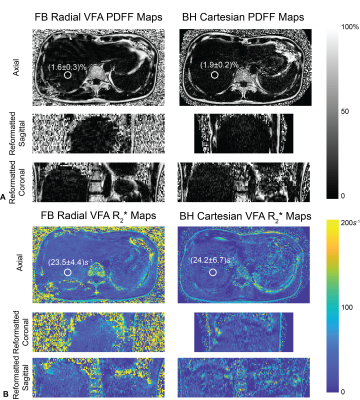
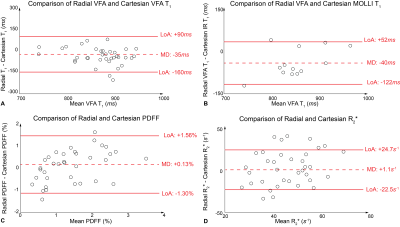
Fig.2 shows T1 maps obtained on a slice imaged by all protocols in one subject. Fig.3 shows PDFF and R2* maps acquired in the same subject using Cartesian VFA and radial VFA. T1 from Cartesian VFA and radial VFA had a mean difference (MD) of 35ms and 95% limits of agreement (LoA) of [-160ms, 90ms] (Fig.4A). T1 from Cartesian MOLLI and radial VFA had a MD of 40ms and LoA of [-122ms, 52ms] (Fig.4B). Cartesian VFA and radial VFA had a MD of 0.13% and LoA of [-1.30%, 1.56%] in PDFF (Fig.4C), while R2* measurements had a MD of 1.1s-1 and LoA of [-22.5s-1, 24.7s-1] (Fig.4D). In repeated radial VFA scans, ICC for T1, PDFF and R2* were 0.896, 0.843 and 0.771, respectively.Discussion
The proposed radial VFA sequence can simultaneous quantify T1, PDFF and R2* in the liver with 3D coverage in a 3-minute free-breathing scan. The built-in motion tracking capability enables reconstructing images at end-expiration to align images with B1+ maps for accurate VFA T1 quantification, and to reduce the effect of motion on R2* mapping. In vivo liver scans demonstrated good repeatability of radial VFA for all parameters despite respiratory motion and potential bulk motion. T1 measured by all three protocols showed good agreement. PDFF showed good agreement between radial and Cartesian VFA. R2* had relatively wide LoA between radial and Cartesian VFA protocols because Cartesian R2* maps had higher levels of noise, likely due to residual motion and parallel imaging artifacts. The radial VFA acquisition time could be further reduced by incorporating parallel imaging techniques20. The new technique can be potentially used as a multiparametric diagnostic tool for liver diseases, and more evaluation is needed in patients with a wider range of liver T1, PDFF, and R2*.Conclusion
We have demonstrated that a new VFA golden-angle-ordered stack-of-radial free-breathing liver MRI method can achieve simultaneous T1, PDFF, and R2* quantification in 3 minutes, with good intra-session repeatability and agreement with reference breath-holding methods.Acknowledgements
This study was supported in part by Siemens Healthineers and UCLA Radiological Sciences. The authors thank the study coordinators at UCLA.References
1. Hoad CL, Palaniyappan N, Kaye P et al. A study of T1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015;28(6):706-714.
2. Banerjee R, Pavlides M, Tunnicliffe EM et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60(1):69-77
3. Li Z, Sun J, Hu X et al. Assessment of liver fibrosis by variable flip angle T1 mapping at 3.0T. J Magn Reson Imaging. 2016;43(3):698-703
4. Tang A, Tan J, Sun M et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422-431
5. Armstrong T, Martin T, Stemmer A et al. Free-breathing fat quantification in the liver using a multiecho 3D stack-of-radial technique: investigation of motion compensation and quantification accuracy. Proc. ISMRM. Paris, France 2017;p0363
6. Idilman IS, Aniktar H, Idilman R et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267(3):767-775
7. Wood JC, Enriquez C, Ghugre N et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(10):1460-1465
8. Tamada D, Wakayama T, Onishi H et al. Multiparameter estimation using multi-echo spoiled gradient echo with variable flip angles and multicontrast compressed sensing. Magn Reson Med. 2018;80(4):1546-1555
9. Zhong X, Armstrong T, Nickel MD et al. Effect of respiratory motion on free-breathing three-dimensional stack-of-radial liver R2* relaxometry and improved quantification accuracy using self-gating. Magn Reson Med. 2019
10. Armstrong T, Dregely I, Stemmer A et al. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn Reson Med. 2018;79(1):370-382
11. Cheng JY, Zhang T, Ruangwattanapaisarn N et al. Free-breathing pediatric MRI with nonrigid motion correction and acceleration. J Magn Reson Imaging. 2015:42(2):407-420
12. Li F, Axel L, Chandarana H et al. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016:75(2):775-788
13. Zhang T, Cheng JY, Chen Y et al. Robust self-navigated body MRI using dense coil arrays. Magn Reson Med. 2016:76(1):197-205
14. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43(5):682-690
15. Deoni SC, Peter TM, Rutt BK. High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med. 2005;53(1):237-241
16. Ren J, Dimitrov I, Sherry AD et al. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2018;49(9):2055-2062
17. Hernando D, Kellman P, Haldar JP et al. Robust water/fat Separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63(1):79-90
18. Messroghli DR, Radjenovic A, Kozerke S et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141-146
19. Chung S, Kim D, Breton E et al. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med. 2011;64(2):439-446
20. Uecker M, Lustig M. Estimating absolute-phase maps using ESPIRiT and virtual conjugate coils. Magn Reson Med. 2017;77(3):1201-1207
Figures