0878
Motion-Resolved, 3D Whole-Brain Simultaneous T1, T2, and T1ρ Mapping using Multitasking with Application to Multiple Sclerosis: A Pilot Study1Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Quantitative multi-parametric relaxometry MRI (e.g., T1, T2, and T1ρ mapping) can demonstrate longitudinal brain changes and enhance lesion contrasts against normal appearing matters in multiple sclerosis. Conventional methods that quantify these relaxation parameters are time-consuming and subject to motion, thus challenging for clinical practice. We present a novel approach that simultaneously quantifies T1, T2, and T1ρ with whole brain coverage in 9min, using the recently developed Multitasking framework that models the multidimensional image as a low-rank tensor. This technique is validated on healthy volunteers. We also demonstrate the feasibility of lesion characterization on relapsing remitting multiple sclerosis patients.
Introduction
Quantitative T1/T2 mapping are capable of abnormal tissue characterization and longitudinal monitoring in multiple sclerosis (MS)1-4. T1ρ, as an emerging contrast mechanism useful for studying low-motional biological processes, demonstrates better enhanced lesion contrast against normal appearing white/gray matters (NAWM/NAGM) than T2 in MS5. Traditional T1/T2/T1ρ mapping methods are inefficient for clinical practice and may produce misaligned maps due to inter-scan motion. Moreover, intra-scan head movement may occur frequently and introduce severe motion artifacts. We propose a novel approach that simultaneously quantifies T1/T2/T1ρ with whole-brain coverage in 9min and can resolve head motion, using the recently developed Multitasking framework to model the multidimensional image as a low-rank tensor (LRT)6. We validate this approach on healthy volunteers with rotational head motion and demonstrate clinical feasibility of tissue characterization on relapsing remitting MS (RRMS) patients.Methods
Sequence Design: The pulse sequence is extended from our previous cardiac/carotid applications6-7, adding T1ρ-preparations to the Multitasking framework for the first time. Specifically, T1/T2/T1ρ contrasts were generated by cycling through 4 B0- and B1-insensitive T2-IR preparations8 (durations $$$\tau$$$=15, 35, 60, 80ms) and T1ρ-IR preparations9 (spin-lock times $$$TSL$$$=15, 40, 65, 90ms, spin-lock frequency=500Hz). Two sets of data were collected with 3D segmented FLASH readouts (flip angle=5$$$^{\circ}$$$, TR/TE=9.4/4.9ms): imaging data ($$$\mathbf{d}_{\mathrm{img}}$$$) were sampled with randomized Cartesian trajectory following Gaussian density along phase and partition encoding directions, and training data ($$$\mathbf{d}_{\mathrm{tr}}$$$) were sampled every 8 readouts at k-space center10. The resulting signal equation is:$$S_{n}=A\cdot\frac{1-e^{-\frac{TR}{T1}}}{1-e^{-\frac{TR}{T1}}\cos(\alpha)}\cdot[1+(Be^{-\frac{\tau}{T2}}e^{-\frac{TSL}{T1\rho}}-1)(e^{-\frac{TR}{T1}}\cos(\alpha))^{n}]\cdot\sin(\alpha),$$where $$$A$$$ absorbs proton density, overall B1 receive field, and T2* weighting, $$$n$$$ represents readout index since preparation, $$$\alpha$$$ denotes FLASH flip angle, and $$$B$$$ represents the effect of inversion efficiency.Image Model: The underlying 7D image $$$I(\mathbf{r},n,s,\tau,TSL)$$$ with spatial locations $$$\mathbf{r}=(x,y,z)$$$, inversion recovery time index $$$n$$$ (same as readout index), motion states $$$s$$$, T2-preparation index $$$\tau$$$, and T1ρ-preparation index $$$TSL$$$ can be modeled as a 5-way LRT $$$\mathcal{X}$$$ with tensor entries $$$X_{ijklm}=I(r_{i},n_{j},s_{k},\tau_{l},TSL_{m})$$$. The image model can be expressed via unfolded tensor factorization:$$\mathbf{X}_{(1)}=\mathbf{U}_{\mathrm{r}}\mathbf{\Phi},$$$$\mathbf{\Phi}=\mathbf{C}_{(1)}(\mathbf{U}_{\mathrm{TSL}}\otimes\mathbf{U}_{\mathrm{\tau}}\otimes\mathbf{U}_{\mathrm{s}}\otimes\mathbf{U}_{\mathrm{n}})^{T},$$where $$$\mathbf{X}_{(1)}$$$ and $$$\mathbf{C}_{(1)}$$$ are mode-1 unfolding of $$$\mathcal{X}$$$ and the core tensor, and the columns of each $$$\mathbf{U}$$$ are basis functions for the corresponding tensor dimensions.
Image Reconstruction: To identify motion states, we perform $$$K$$$-means clustering of the subset of $$$\mathbf{d}_{\text{tr}}$$$ corresponding to the last $$$n_{j}$$$ of each shot. To select the number of motion states/clusters $$$K$$$, the algorithm is performed for $$$K$$$=1,2,…,10, choosing the elbow of the Euclidean distance plot as the final $$$K$$$. To reduce the effect of intra-bin motion during the transition between motion states, we calculate a diagonal motion-weighting matrix $$$\mathbf{W}$$$ from training data residual $$$\mathbf{R}$$$:$$\mathbf{R}=\mathbf{D}_{\mathrm{tr}}-\mathbf{D}_{\mathrm{tr}}\mathbf{\Phi}_{\mathrm{tr}}^{\dagger} \mathbf{\Phi}_{\mathrm{tr}},$$$$W_{jj}=\left(\sum_{i}\left|R_{ij}\right|^{2}\right)^{-1/2},$$where $$$\mathbf{D}_{\mathrm{tr}}$$$ is the Casorati matrix11 of $$$\mathbf{d}_{\mathrm{tr}}$$$, and $$$\mathbf{\Phi}_{\mathrm{tr}}$$$ contains “real-time” temporal factors (i.e., only a single, elapsed time dimension indexing total number of time points) extracted from SVD of $$$\mathbf{D}_{\mathrm{tr}}$$$. Following that, the multidimensional tensor basis functions $$$\mathbf{\Phi}=\mathbf{C}_{(1)}(\mathbf{U}_{\mathrm{TSL}}\otimes\mathbf{U}_{\mathrm{\tau}}\otimes\mathbf{U}_{\mathrm{s}}\otimes\mathbf{U}_{\mathrm{n}})^{T}$$$ are estimated via motion-weighted Bloch-constrained LRT completion6 of motion-weighted $$$\mathbf{d}_{\mathrm{tr}}$$$. Lastly, the spatial factor $$$\mathbf{U_{\mathrm{r}}}$$$ is obtained by:$$\mathbf{U}_{\mathrm{r}}=\arg\min_{\mathbf{U}_{\mathrm{r}}}\|\mathbf{W}[\mathbf{d}_{\mathrm{img}}-E(\mathbf{U}_{\mathbf{r}}\Phi)]\|^{2}+R_{s}(\mathbf{U}_{\mathrm{r}}),$$where $$$E(\cdot)$$$ combines multichannel encoding and sampling, and $$$R_{s}(\cdot)$$$ performs spatial regularization.
Experiment Design: Data were collected on a 3T scanner (Biograph mMR, Siemens) on n=7 healthy subjects and n=3 RRMS patients. Reference methods for healthy subjects included: 1) inversion-recovery-turbo-spin-echo for T1; 2) multi-echo-spin-echo for T2; 3) T1ρ-prepared-FLASH for T1ρ. The Multitasking sequence was run twice on healthy volunteers to test repeatability. One volunteer was instructed to perform rotational motion once without returning to the original position, with in-plane rotation angle >30$$$^{\circ}$$$. For RRMS patients, only the Multitasking sequence was run during clinical scans. Scan time of healthy volunteers was 26min for references and 9min for Multitasking. Multitasking scan parameters were: FOV=240x240x140mm3, resolution=1x1x3.5mm3.
Image Analysis: T1/T2/T1ρ measurements are evaluated. The quantitative agreement between Multitasking and references is assessed with intra-class correlation coefficients (ICC). WM/GM segmentation is performed by manually thresholding the respective target images.
Results
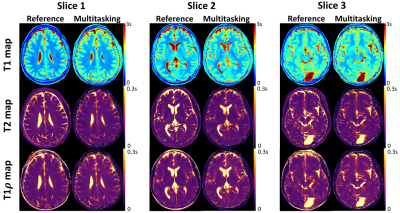
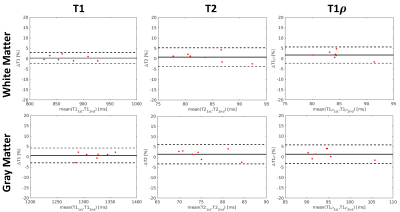
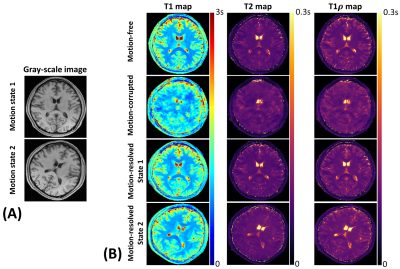
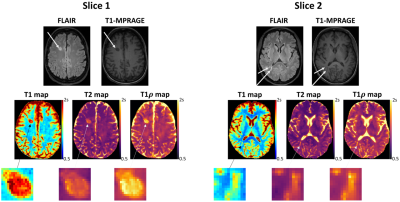
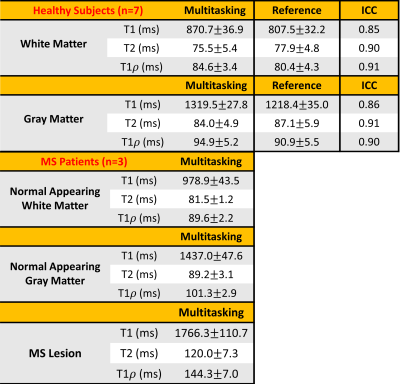
High-quality, co-registered T1/T2/T1ρ maps are generated with Multitasking and show consistency with reference maps (Fig.1). T1/T2/T1ρ measurements demonstrate good repeatability between the first and second Multitasking scans on healthy volunteers (Fig.2). The Multitasking framework demonstrates excellent motion-resolved ability, yielding clean T1/T2/T1ρ maps comparable to those from motion-free scan, while without motion-resolving ($$$K$$$=1), T1/T2/T1ρ maps are severely motion-corrupted (Fig.3). MS lesions are clearly shown on Multitasking T1/T2/T1ρ maps, which is consistent with qualitative clinical images (Fig.4). Multitasking T1/T2/T1ρ values of WM/GM show excellent quantitative agreement with reference values for healthy volunteers with all ICC$$$\geq$$$0.85, while MS lesions and NAWM/NAGM in patients have substantially higher T1/T2/T1ρ values than WM/GM of normal controls (Table 1).Discussion
The Multitasking framework generates co-registered T1/T2/T1ρ maps, produces repeatable measurements that agree with reference measurements, and resolves head motion to produce artifact-free maps. WM/GM of healthy controls, MS lesions and NAWM/NAGM of RRMS patients are distinguishable from each other. Although NAWM/NAGM are “normal appearing” in qualitative images, they are differentiable from healthy control tissue in quantitative maps.Conclusion
We propose a novel framework for motion-resolved, simultaneous whole-brain T1/T2/T1ρ quantification in 9min. High-quality, co-registered, and motion-resolved T1/T2/T1ρ maps are presented which are free from motion artifacts. T1/T2/T1ρ values are in excellent agreement with references and show good repeatability. Clinical pilot studies are conducted on MS patients, demonstrating the ability of lesion and tissue characterization. Comprehensive clinical validation will be conducted in future work.Acknowledgements
This work was supported by NIH 1R01EB028146.References
1. Vrenken H, Rombouts SARB, Pouwels PJW, Barkhof F. Voxel-based analysis of quantitative T1 maps demonstrates that multiple sclerosis acts throughout the normal-appearing white matter. AJNR, 2006; 27(4): 868-874.
2. Seewann A, Vrenken H, van der Valk P, Blezer ELA, Knol DL, Castelijn JA, et al. Diffusely abnormal white matter in chronic multiple sclerosis: imaging and histopathologic analysis. Arch. Neurol, 2009; 66(5): 601-609.
3. Bonnier G, Maréchal B, Fartaria MJ, Falkowskiy P, Marques JP, Simioni S, et al. The combined quantification and interpretation of multiple quantitative magnetic Resonance imaging metrics enlightens longitudinal changes compatible with brain repair in relapsing-remitting Multiple Sclerosis patients. Front Neurol, 2017; 8:506.
4. Blystad I, Håkansson I, Tisell A, Ernerudh J, Smedby Ö, Lundberg P, et al. Quantitative MRI for analysis of active multiple sclerosis lesions without gadolinium-based contrast agent. AJNR, 2016; 37(1): 94-100.
5. Gonyea, JV, Watts R, Applebee A, Andrews T, Hipko S, Nickerson JP, et al. In vivo quantitative whole‐brain T1ρ MRI of multiple sclerosis. JMRI, 2015; 42(6): 1623-1630.
6. Christodoulou AG, Shaw JL, Nguyen C, Yang Q, Xie YB, Wang N, et al. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng. 2018;2(4):215-226.
7. Xie Y, Christodoulou AG, Wang N, Li D. Quantitative Multi-Contrast Atherosclerosis Characterization (qMATCH): Comprehensive Quantitative Evaluation of Atherosclerosis in a Single-Scan. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, HI, 2017. p. 3122.
8. Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1‐insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med, 2006; 55(4): 858-864.
9. Mitrea, BG, Krafft, AJ, Song R, Loeffler RB, Hillenbrand CM. Paired self-compensated spin-lock preparation for improved T1ρ quantification. JMR 2016; 268: 49-57.
10. Ma S, Nguyen C, Han F, Wang N, Deng Z, Binesh N, et al. Three-Dimensional Simultaneous Brain T1, T2, and Apparent Diffusion Coefficient Mapping with MR Multitasking. Magn Reson Med, 2019. In press.
11. Liang Z-P. Spatiotemporal imaging with partially separable functions. In Proceedings of the 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Arlington, Virginia, USA, 2007. P. 988-991.
Figures