0877
Fast Simultaneous T1, T2 and T2* Mapping at High Spatial Resolution using 3D Echo-planar Time-resolved Imaging (3D-EPTI)1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 33Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States
Synopsis
An efficient quantitative mapping sequence based on 3D Echo-Planar Time-resolved Imaging (3D-EPTI) is proposed. The acquisition contains inversion recovery gradient echo readouts follow by GRASE-like readouts to provide sensitivity to T1, T2 and T2*. Fast k-TI-TE coverage is achieved by fusing highly-accelerated spatiotemporal CAIPI sampling with golden-angle radial-blade Cartesian under-sampling, where the reconstruction is performed using the subspace model. We demonstrate the high-efficiency of the proposed technique by obtaining multi-contrast images and quantitative maps at 1-mm isotropic resolution whole-brain in 3 minutes.
Introduction
MR quantitative imaging is challenging: the acquisition of a series of images with different contrast weightings is usually required to capture the signal decay/recovery evolution, resulting in extensive acquisition time. With the advent of acceleration techniques such as parallel imaging1,2 and compressed sensing3,4, it has become more and more clinically feasible, but the combined scan time for T1, T2, T2* mapping is still in the order of 10-20 minutes with limited image quality.In this work, we developed a new technique, termed 3D echo-planar time-resolved imaging (3D-EPTI), that takes advantages of i) the high-efficiency of EPI readout; ii) spatial-temporal correlation across EPI readout; and iii) low-rank subspace reconstruction, in order to achieve rapid simultaneous T1, T2, T2* mapping at high-resolution with high SNR-efficiency. With 3D-EPTI, a time-series of distortion and blurring-free images are produced which captures the MR signal evolution during the acquisition. This signal evolution has been designed to provide high sensitivity to MR relaxometry differences by utilizing an inversion recovery pulse followed by small flip angle pulse train along with a GRASE readout. We demonstrate the high efficiency of this technique in obtaining multi-contrast images and quantitative maps at 1-mm isotropic resolution whole brain coverage in 3 minutes.
Methods
3D EPTI readout: In EPTI5, continuous EPI-readouts are performed using highly-accelerated k-t CAIPI-sampling to efficiently sample the desired signal evolution in k-t space. As shown in Fig.1a for 3D-EPTI, each EPTI-shot covers a block of the ky-kz-te space using a zig-zag trajectory that samples complementary ky and kz along TE. This ensures that the neighboring k-points within each EPTI-shot are acquired within a few milliseconds apart and contain high signal-correlation to help reconstruct the missing k-t data-points. This data can then be efficiently reconstructed via subspace reconstruction to generate time-resolve distortion and blurring-free images with a TE increment of ~1ms. Continuous EPTI-readout can be applied to any sequences for high acquisition efficiency without any dead time.Pulse-sequence design (Fig. 1b): The sequence utilizes an inversion recovery pulse followed by a small-flip-angle GE train (IR-GE) to provide signal-evolution with T1 recovery interspersed with T2* decay. Subsequent to the small-flip-angle train, an additional GRASE-like acquisition can be appended to provide additional signal evolution with T2 and T2* decay. Each excitation/refocusing pulse is followed by an 3D EPTI readout that covers a block of the 3D k-space to track the signal evolution. However, acquiring the full-block 3D k-space for each time point would require lengthy acquisition time. Therefore, we developed golden angle radial-blade sampling, where different 3D-EPTI blocks acquired across multiple TRs at the same TI form a diagonal radial-blade in ky-kz space, and different blade angulations at different TIs compose a golden-angle radial-blade Cartesian sampling pattern (Fig.1b bottom). This creates favorable spatio-temporal incoherent aliasing for constrained reconstruction and permits further acceleration through acquiring only 1-2 blade per time point.
Subspace reconstruction: A subspace reconstruction6 is used to recover the images at different time points, which relays on the subspace bases extracted from possible signal evolution curves generated using tissue and acquisition parameters. A Hankel constrain is employed in the reconstruction to improve the conditioning and SNR. A pixel-wise matching is used to obtain the quantitative maps.
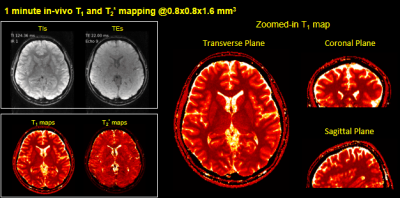
Experiments: In-vivo retrospective and prospective undersampling experiments were performed where 3D-EPTI with IR-GE was used to obtain T1 and T2* maps. i) In retrospective experiment, EPTI data across all ky-kz blocks were acquired (20.5 minutes, at 1.1 mm isotropic resolution) and retrospectively undersampled with the proposed golden angle sampling pattern with a single blade to achieve an effective ~1minute scan time. In prospective experiment, data were acquired at 0.8×0.8×1.6mm3 resolution in 1-minute with whole-brain coverage (FOV =220×220×116 mm3).
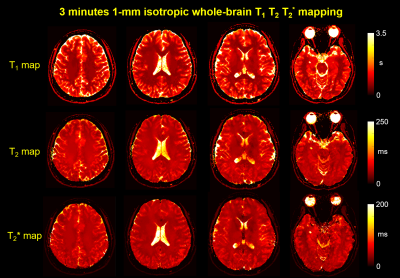
ii) In vivo simultaneous T1, T2 and T2* prospective undersampling experiment was performed using IR-GE-GRASE 3D-EPTI. Data were acquired at 1mm isotropic resolution in ~3 minutes with 2-radial blades. Non-selective RF-pulse was used to achieve large coverage with readout direction along head-foot. 26 TIs (each with 53 TEs) and 6 GRASE (each with 39 TEs) time-points were acquired. Other imaging parameters: FOV =224×180×224mm3, Ryblock×Rzblock=8×6; full block-sampling=29×29=841 blocks, dual-blade sampling=29×2 blocks; Total acceleration=8×6×29.
Results
Fig.2a shows that 1-minute IR-GE 3D-EPTI provide comparable quantitative T1 and T2* maps to block-fully-sampled EPTI data that requires 19× longer scan time, with consistent quality in all three orthogonal planes (Fig.2b). The GIF in Fig.3 shows the multi-contrast images across different TIs and TEs acquired in prospective experiment with IR-GE sequence in only 1-minute, along with T1 and T2* maps across different slices. The whole-brain zoomed-in T1 map illustrates the high-quality of the acquired quantitative measurements. Fig.4 and Fig.5 show the results of in-vivo experiment using the IR-GE-GRASE sequence. Thousands of whole-brain images were obtained in only 3 minutes with different contrasts to track the signal evolution, and high-quality quantitative maps including T1, T2 and T2* are calculated and presented in Fig.5.Conclusion
The high acquisition efficiency provided by 3D EPTI enables fast and comprehensive quantitative mapping in 3 minutes at 1-mm isotropic resolution whole-brain. Future work will focus on further validation and optimization of this technique to push the clinical application of quantitative mapping using 3D-EPTI.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB020613, R01-EB019437, R01-MH116173, U01-EB025162, and U01EB026996) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952-962.
2. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202-1210.
3. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182-1195.
4. Huang C, Graff CG, Clarkson EW, Bilgin A, Altbach MI. T2 mapping from highly undersampled data by reconstruction of principal component coefficient maps using compressed sensing. Magn Reson Med. 2012;67:1355-1366.
5. Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med. 2019;81:3599-3615.
6. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-Resolved Imaging (EPTI) with Subspace Reconstruction and Optimized Spatiotemporal Encoding. arXiv preprint arXiv:2019; 1911.00999..
Figures