0875
3D UTE-MRF for multiple parametric maps with sub-millimeter isotropic resolution using multi-dimensional golden-angle radial trajectory1Center for Brain Imaging Science and Technology, Key Laboratory for Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, China, 2Siemens Healthcare Ltd., Shanghai, China, 3State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, China
Synopsis
In this study, we used a 3D ultrashort-echo-time MR Fingerprinting (UTE-MRF) method to generate distortion-free quantitative T1, T2, and proton density maps with an isotropic resolution of 0.8 x 0.8 x 0.8 mm3.
Introduction
Image distortion around orbital prefrontal lobe and cranial base in ultrashort-echo-time (UTE) imaging is minimized by using a sub-millisecond echo time (TE) and short readout period. The 2D ultrashort-echo-time MR fingerprinting method was proposed to simultaneously quantify T1, T2, and PD maps for long and ultrashort T2 tissues in our previous study.1 However, limited by the half-pulse slice excitation scheme, whole imaging volume coverage with an isotropic resolution could not be achieved due to the RF power and gradient amplitude limitations. In this work, a 3D UTE-MRF method is presented for whole volume coverage with a submillimeter isotropic resolution in the brain and knee.Methods
Our previous 2D UTE-MRF method 1 was extended to 3D. The sequence diagram is shown in Figure 1. For each repetition, the flip angle (FA) varied from 5° to 60° in a multiple-half-sinusoidal pattern to model T1 and T2 into signal changes. 1 A hard pulse with a duration of 0.2 ms was utilized for volume excitation. With a center-out dual-echo radial readout, TE was minimized and set to a fixed 0.15 ms. TE for the second echo was about 2 ms, and TR was fixed to 7 ms. The sequence was repeated multiple times in conjunction with a sliding-window reconstruction algorithm 2 to reduce the undersampling rate for each frame. An interval of 2 seconds was set between repetitions to recover the longitudinal magnetizations.An optimized multi-dimensional golden-angle method 3 was employed to cover the 3D k-space and maximize the signal incoherence between adjacent frames. The k-space coverage of the first frame under repetitions of 160 is shown in Figure 1C. Point spread functions are shown on the right side, which have been reconstructed using a single frame and a combination of 40 frames (i.e., window size of 40), respectively. The main lobes were narrowed, and the side lobes were decreased under the window size of 40, which could be noticed at the 1D plots. The total acquisition time was 16 mins.
Experiments were performed on a 3T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) using a 64-channel head coil and 15-channel knee coil. k-Space spokes at the same frames were reconstructed into a 256 x 256 x 256 matrix under the resolution of 0.8 x 0.8 x 0.8 mm3 using NUFFT.4 Dictionary matching was performed after a sliding-window combination using a window size of 40.
Results
Figure 2 shows the quantification maps of the knee from the first echo of 3D UTE-MRF. Cortical bone is enhanced in the last row by a bone-enhanced algorithm. 1Figure 3 shows the comparisons of T1 and T2 maps between the results from echo 1 and echo 2. White matter and gray matter show distinct differences in T1 value.
Figure 4 shows T1, T2, and PD maps from three orthogonal planes reconstructed of the brain using echo 1. 3D UTE MRF produces full coverage of head and neck. Signal at nasal cavity was largely restored, and almost no distortion was observed.
Discussion
MRF simultaneously quantifies multiple parameters by modeling tissue properties into the undersampled signal evolution. Dramatically increased undersampling aliasing degrades the quantification accuracy when extending a 2D MRF method into 3D. Solutions have focused on using either advanced reconstruction methods, or the enlarged k-space coverage. 5, 6 In the proposed 3D UTE-MRF method, a radial trajectory with a short readout period was used to reduce the T2* blurring. Thus, a radial spoke has 4 ~ 10 times the undersampling rate of a spiral arm. It is inefficient to acquire the same amount of data set as a spiral readout by prolonging the imaging time by 4 ~ 10 times. A multidimensional golden angle method was optimized by Zhang et. al. to cover k-space efficiently and tested in lung imaging.3 In the 3D UTE-MRF, the multi-dimensional golden-angle method was employed to maximize the k-space coverage and data incoherence for adjacent frames.The 3D UTE-MRF method can simultaneously obtain T1, T2, and PD maps at two echo times. Because short T2 components are acquired in the first echo, bone-enhanced images can be reconstructed: These are shown in Figure 2. The 3D UTE-MRF method can achieve whole brain and neck coverage because image distortions within the nasal cavity, prefrontal lobe, and cranial base are minimized with ultrashort TE and short readout period. A significant difference between white matter and gray matter in T1 maps from the two echoes was observed in the Figure 3 and may be caused by the short T2 component in white matter and partial-volume effect from CSF in gray matter.7, 8
Conclusions
A 3D UTE-MRF method was proposed to achieve distortion-free multiple parametric maps with 0.8 x 0.8 x 0.8 mm3 isotropic resolution and whole volume coverage. This method could significantly reduce susceptibility artifacts and may also be applied to ultra-high-field tissue mapping.Acknowledgements
No acknowledgement found.References
Li Q, Cao X, Ye H, et al. Ultrashort echo time magnetic resonance fingerprinting (UTE‐MRF) for simultaneous quantification of long and ultrashort T2 tissues. Magnetic Resonance in Medicine, 2019, 82(4): 1359-1372.
Cao X, Liao C, Wang Z, Chen Y, Ye H, He H, Zhong J. Robust sliding-window reconstruction for Accelerating the acquisition of MR fingerprinting. Magn. Reson. Med. 2017; 78(4): 1579–1588.
Zhang J., Feng L., Otazo R., et al. Rapid dynamic contrast-enhanced MRI for small animals at 7T using 3D ultrashort echo time and golden-angle radial sparse parallel MRI. Magn Reson Med, 2019, 81(1): 140-152.
Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans. Signal Process. 2003;51:560-574.
Liao C.Y., Bilgic B., Manhard M.K., et al. 3D MR fingerprinting with accelerated stack-of-spirals and hybrid sliding-window and GRAPPA reconstruction. Neuroimage, 2017, 162: 13-22.
Cao X, Ye H, Liao C, et al. Fast 3D brain MR fingerprinting based on multi‐axis spiral projection trajectory. Magnetic Resonance in Medicine, 2019, 82(1): 289-301.
Horch R A, Gore J C, Does M D. Origins of the ultrashort‐T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magnetic resonance in medicine, 2011, 66(1): 24-31.
Fan S J, Ma Y, Chang E Y, et al. Inversion recovery ultrashort echo time imaging of ultrashort T2 tissue components in ovine brain at 3 T: a sequential D2O exchange study. NMR in Biomedicine, 2017, 30(10): e3767.
Figures
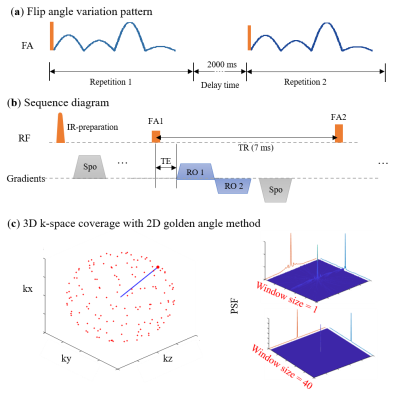
Figure 1. Sequence diagram of 3D dual-echo UTE-MRF. (a) FA variation pattern. There is an interval of 2 s between each repetition. (b) Sequence diagram in a TR. (c) 3D k-space coverage of frame 1 using a multi-dimensional golden-angle method and corresponding point spread functions.
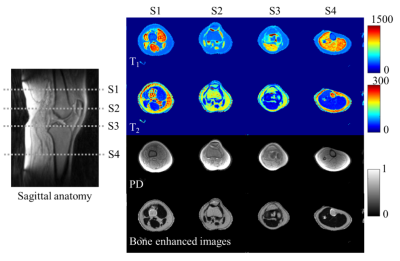
Figure 2. T1, T2, and PD maps of knee from the first echo of 3D UTE-MRF. Cortical bone is enhanced in the last row. Slice positions are labeled in a sagittal view of the imaging volume. T2 value of fat in S1 and S4 differs from that in S2 and S3 due to B1 inhomogeneity.
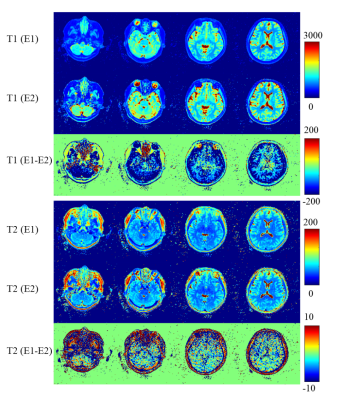
Figure 3. T1 and T2 maps reconstructed from echo 1 and echo 2 and their differences. T1 and T2 maps from the second echo show lower SNR than that from the first echo. White matter and gray matter show significant differences in T1 map between two echoes, little such regional differences are seen in T2 maps.
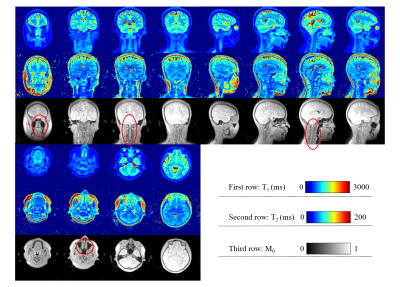
Figure 4, T1, T2, and M0 maps of three orthogonal planes. These images were reconstructed from echo 1. Little distortion or signal blurring could be seen even within nasal cavity, prefrontal lobe, and cranial base (marked by red circles).