0872
Generalised Low-Rank Non-rigid Motion Corrected reconstruction for 2D Cardiac MRF1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Philips Healthcare, Guildford, United Kingdom
Synopsis
Cardiac Magnetic Resonance Fingerprinting (cMRF) has been proposed for simultaneous myocardial T1 and T2 mapping. This approach uses ECG-triggering to synchronize data acquisition to a small mid-diastolic window, reducing cardiac motion artefacts but also limiting the amount of acquired data per heartbeat. This low scan efficiency can limit the spatial resolution achievable in a breath-held scan. Here we introduce a novel approach for contrast-resolved motion-corrected reconstruction, that combines the generalized matrix description formulism for non-rigid motion correction with low-rank compression of temporally varying contrast. This approach enables longer acquisition windows and higher scan efficiency in cMRF, correcting for cardiac motion.
INTRODUCTION:
Cardiac Magnetic Resonance Fingerprinting1 (cMRF) enables simultaneous and co-registered myocardial T1 and T2 mapping in a single breath-hold scan. This approach uses ECG-triggering to synchronize data acquisition to a small mid-diastolic window (~200ms), reducing cardiac motion artefacts but also limiting the amount of acquired data per heartbeat. This low scan efficiency can limit the spatial resolution achievable in a breath-held scan and/or result in long acquisition times. Here we propose to double the cMRF acquisition window for improved scan efficiency by integrating non-rigid cardiac motion correction directly in the reconstruction of the contrast-resolved images. This is achieved with a so-called Low Rank Motion Corrected reconstruction (LR-MC), combining elements of low rank modelling2,3,4 (to resolve contrast) and dense motion fields5,6,7 (for elastic motion correction). Preliminary evaluation of the proposed approach was performed in four healthy subjects in comparison to non-motion corrected low rank MRF.METHODS:
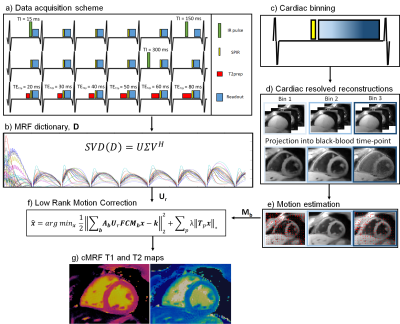
In the proposed framework 2D cMRF data is acquired in a similar fashion to previous ECG-triggered 2D/3D cMRF approaches1,9. Different inversion recovery (IR) and T2 preparation (T2prep) pulses are applied at each heartbeat to encode T1 and T2, whereas a spectral presaturation with IR (SPIR) pulse is used before data acquisition to minimise aliasing artefacts originating from fat signal (Fig.1a). Different to previous approaches, a long acquisition window of ~425 ms is employed here. The reconstruction framework proposed here consists of two parts: 1) motion resolved reconstruction for non-rigid cardiac motion estimation, and 2) LR-MC reconstruction. Motion estimation: data is binned into three cardiac bins of equal size; each cardiac phase is reconstructed with Low Rank Inversion (LRI4) regularized with HDPROST10 independently, producing a set of cardiac motion resolved singular images (Fig.1c). Singular images from each cardiac phase are then decompressed to the time-points domain and the second heartbeat after the first inversion recovery, where image contrast is approximately black-blood11 (Fig1.d), is used to estimate non-rigid cardiac motion via image registration12 (Fig.1e). LR-MC reconstruction (Fig.1f): the estimated motion fields for each cardiac bin b are then incorporated into the proposed low rank motion corrected reconstruction with HDPROST regularization, formulated as:$$\bf{x} = argmin_x \parallel \bf{\sum_b A_bU_rFCM_bx-k} \parallel _2^2 + \lambda \sum_p \parallel \bf{T_px} \parallel _*$$
where, $$$U_r$$$, $$$F$$$ and $$$C$$$ are dictionary-based compression, non-uniform Fast Fourier Transform and coil sensitivity operators; $$$x$$$ are the temporally compressed singular images for the cardiac motion corrected MRF data, $$$k$$$ is the acquired k-space, and $$$A_b$$$ and $$$M_b$$$ are respectively the sampling matrices and the sparse motion matrices for the b-th motion state. For HDPROST regularization, $$$T_p$$$ constructs 3D local tensor around each voxel p in the motion corrected image by concatenating local (within a patch), non-local (between similar patches) and contrast (along the singular value domain) voxels along each dimension. T1 and T2 maps (Fig.1g) are obtained from the LR-MC reconstructed singular images via MRF template matching.
EXPERIMENTS:
Four healthy subjects were scanned at a 1.5T scanner (Philips Ingenia). Imaging parameters included field of view (FOV) = 315x315 mm2; 8 mm slice thickness; resolution = 1.75x1.75 mm2; TE/TR = 0.9/7.1 ms; gradient echo readout; 6-10º sinusoidally varying flip angle; acquisition window = 425 ms; 1080 time-points; nominal scan time 18s. Acquired data was reconstructed with the proposed LR-MC and without motion correction via LRI-HDPROST (i.e. omitting steps c),d) and e) in Fig.1) applied to the full acquisition window.RESULTS:
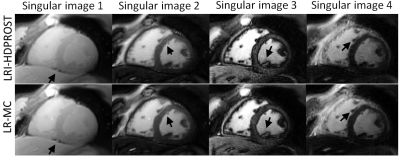
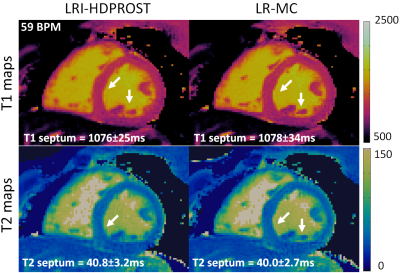
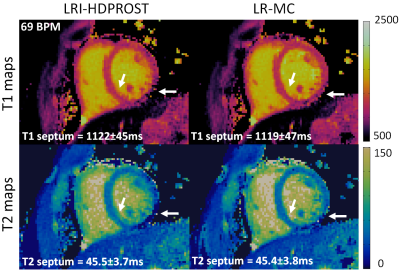
Residual motion artefacts observed in the singular images obtained with non-motion corrected LRI-HDPROST were reduced with the proposed LR-MC approach (Fig.2). Motion artefacts in non-motion corrected LRI-HDPROST propagated to the resulting T1 and T2 maps, whereas these artefacts were reduced with the proposed LR-MC reconstruction (Fig.3) in both T1 and T2 maps, improving delineation of the myocardium and papillary muscles (arrows). T1 and T2 maps for a second representative subject are shown in Fig.4. Significant motion artefacts are observed with non-motion corrected LRI-HDPROST, particularly in the infero-lateral wall of the myocardium. The proposed LR-MC approach restores the visualization of the infero-lateral wall and improves the overall sharpness of the myocardium and papillary muscles (Fig.4, arrows).CONCLUSION:
A novel contrast resolved, motion corrected reconstruction was proposed and investigated for cardiac motion correction in 2D cMRF with extended acquisition windows (~425 ms). Preliminary results show improved parametric maps for the proposed LR-MC approach and thus the increased scan efficiency could be leveraged into increased resolution or reduced scan time. Future work will investigate further extending the acquisition window and validating the proposed approach in heathy subjects and patients with cardiovascular disease. The proposed contrast resolved, motion corrected LR-MC reconstruction could be applied to other multi-contrast/multi-parametric applications and will be investigated as future work.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC (EP/P001009, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/ A000049/1).References
1. Hamilton JI, Jiang Y, Chen Y, et al. MR fingerprinting for rapid quantification of myocardial T 1 , T 2 , and proton spin density. MRM 2017;77:1446–1458.
2. McGivney DF, Pierre E, Ma D, et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Trans. Med. Imaging 2014;33:2311–2322 doi: 10.1109/TMI.2014.2337321.
3. Zhao B, Setsompop K, Adalsteinsson E, et al. Improved magnetic resonance fingerprinting reconstruction with low-rank and subspace modeling. Magn. Reson. Med. 2018;79:933–942 doi: 10.1002/mrm.26701.
4. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
5. Batchelor PG, Atkinson D, Irarrazaval P, Hill DLG, Hajnal J, Larkman D. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 2005;54:1273–1280 doi: 10.1002/mrm.20656.
6. Odille F, Vuissoz PA, Marie PY, Felblinger J. Generalized reconstruction by inversion of coupled systems (GRICS) applied to free-breathing MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Jul;60(1):146-57.
7. Cruz G, Atkinson D, Buerger C, Schaeffter T, Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magnetic resonance in medicine. 2016 Apr;75(4):1484-98.
8. Pruessmann KP, Weiger M, Börnert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Oct;46(4):638-51.
9. Cruz G, Jaubert O, Schneider T, Bustin A, Botnar Rm, Prieto C. Toward 3D Free-breathing Cardiac Magnetic Resonance Fingerprinting. ISMRM 2019; abstract number 4385.
10. Bustin A, Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn. Reson. Med. 2019:3705–3719 doi: 10.1002/mrm.27694.
11. Becker, KM, Blaszczyk, E, Funk, S, et al. Fast myocardial T1 mapping using cardiac motion correction. Magn Reson Med. 2020; 83(2):438-451. https://doi.org/10.1002/mrm.27935
12. Modat M, Ridgway G, Taylor Z, Lehmann M, Barnes J, Hawkes D, Fox N, Ourselin S. Fast free-form deformation using graphics processing units. Comput Meth Prog Bio 2010; 98: 278– 284.
Figures