0868
Feasibility of MR fingerprinting using a high-performance 0.55T MRI system
Adrienne E. Campbell-Washburn1, Yun Jiang2, Gregor Körzdörfer3,4, Mathias Nittka3, and Mark A. Griswold2
1National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
1National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
Synopsis
We assess the feasibility of T1 and T2 mapping with MR fingerprinting implemented on a high-performance 0.55T system that combines contemporary hardware and imaging methods with a lower magnetic field strength. Quantitative values correlated closely to spin-echo measurements in the NIST phantom. Brain MRF was evaluated in 12 healthy volunteers and liver MRF was evaluated in one volunteer as a proof-of-concept. At 0.55T, T1 was 539ms (white matter) and 660ms (gray matter), and T2 was 64ms (white matter) and 76ms (gray matter). The combination of MRI fingerprinting and low-field MRI systems provides an opportunity for rapid, low-cost, quantitative imaging.
Introduction
We recently described a high-performance low-field MRI system that combines contemporary hardware and imaging methods with a lower magnetic field strength (0.55T) [1]. This system configuration may offer opportunities for cost-efficient imaging, but is also capable of achieving technically demanding applications. Magnetic resonance fingerprinting (MRF) is an established method for rapid and reproducible quantitative MRI [2, 3]. Spiral acquisitions, such as those used for MRF, are particularly amenable to lower field strength because of the prolonged T2* and reduced off-resonance blurring, by virtue of the B0 field homogeneity. The combination of MR fingerprinting and low-field MRI system provides an opportunity for rapid, low-cost, quantitative imaging [4]. In this study, we assess the feasibility of T1 and T2 mapping with MRF at 0.55T.Methods
We used an MRI system modified to operate at 0.55T (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). This system has contemporary gradient performance (45mT/m, 200T/m/s), magnet design, and receiver architecture. 3D MRF was performed using a 48-shot FISP spiral design with 4.6ms spiral readout duration (TE/TR = 1.36/8.88ms, FOV = 300mm x 300mm, in-plane resolution = 1.2mm x 1.2mm, slice thickness = 5mm, 36 slices). Undersampled images were reconstructed using NUFFT, and signal was fit to a simulated signal evolution dictionary with T1 = [10ms, 5000ms] and T2 = [2ms, 1200ms].T1 and T2 values were validated using the NIST quantitative imaging phantom, compared to conventional spin echo measurements with multiple inversion times (TI = 10, 20, 30, 40, 80, 160, 320, 640, 1300, 2600, 5000, 8000ms) and multiple TEs (TE = 10, 20, 40, 80, 160, 320, 640, 1000ms). Healthy volunteer imaging was approved by the local Institutional Review Board and MRF was evaluated in the brain of 12 healthy volunteers using a 16-channel head/neck receive array re-tuned to operate at 0.55T. As a proof-of-concept, one healthy volunteer was imaged to assess liver MRF, using a 6-channel body receive array and 18-channel spine coil re-tuned for 0.55T.
Results
Total acquisition time for a 3D volume was 8:30min. We found T1 and T2 values calculated by MRF were strongly correlated to spin echo measurements in the NIST phantom (R2>0.99, Figure 1). T1 and T2 maps for multiple slices in a healthy volunteer are provided in Figures 2 and 3. Maps were found to have reasonable quality and SNR for isotropic spatial resolution of 1.2mm. No correction for off-resonance blurring was required for spiral readout length of 4.6ms. Mean T1 and T2 values for white matter and gray matter from 12 healthy volunteers are provided in Figure 4. At 0.55T, T1 values were 32% shorter than at 1.5T, and T2 values were similar to MRF measurements at 1.5T [2]. T1 values were comparable to previous in vivo measurements at 0.55T, whereas T2 measured by MRF was shorter [1]. Figure 5 provides example liver MRF results from a single volunteer to illustrate the potential of rapid quantitative evaluation in this more challenging anatomy.Discussion and Conclusion
We demonstrated the feasibility of MRF quantification using a high-performance 0.55T MRI system. The combination of low-field MRI and rapid quantitative imaging with MRF is attractive for efficient, low-cost, clinical evaluation. No off-resonance correction was required for spiral acquisitions at 0.55T, and B0 and B1 homogeneity provided high quality quantitative maps. Future work will further optimize the acquisition for the physical properties of a 0.55T MRI system.Acknowledgements
Funding for this work was provided by the National Heart, Lung and Blood Institute’s Division of Intramural Research. Case Western Reserve University receives funding from Siemens Healthcare. NHLBI and Siemens have a collaborative research agreement for the development of low field MRI and Siemens assisted with the system modification for this 0.55T MRI system.References
- Campbell-Washburn, A.E., et al., Opportunities in Interventional and Diagnostic Imaging by Using High-performance Low-Field-Strength MRI. Radiology, 2019. 293(2): p. 384-93.
- Ma, D., et al., Magnetic resonance fingerprinting. Nature, 2013. 495(7440): p. 187-92.
- Panda, A., et al., Magnetic Resonance Fingerprinting-An Overview. Curr Opin Biomed Eng, 2017. 3: p. 56-66.
- Sarracanie, M., et al., Low-Cost High-Performance MRI. Sci Rep, 2015. 5: p. 15177.
Figures
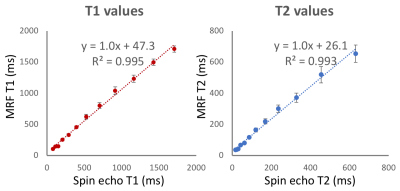
Figure 1: Validation of quantitative T1 and T2 mapping using MRF, compared to spin echo measurements, using the NIST quantitative imaging phantom.

Figure 2: 3D T1 maps in a healthy volunteer generated using MRF at 0.55T.
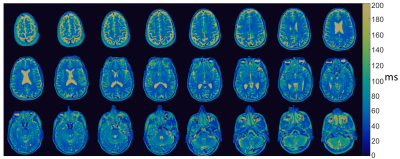
Figure 3: 3D T2 maps in a healthy volunteer generated using MRF at 0.55T.
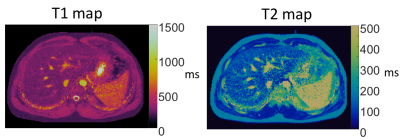
Figure 4: Example liver T1 and T2 maps in a healthy volunteer generated using MRF at 0.55T.

Figure 5: Mean T1 and T2 values in white matter and gray matter calculated by MRF at 0.55T (12 healthy volunteers).