0866
Contribution of parenchymal water and CSF-like water to changes in brain water diffusivity in post-natal development and aging1NIBIB, National Institutes of Health, Bethesda, MD, United States, 2Henry M. Jackson Foundation, Bethesda, MD, United States, 3Alzheimer’s Disease Neuroimaging Initiative, Los Angeles, CA, United States
Synopsis
In this study, we
used a biexponential dual compartment diffusion tensor imaging (DTI) model to
characterize different pools of parenchymal water to investigate CSF-like free
water and parenchymal diffusivity in childhood, early adulthood, and elderly
adulthood, and examine how they change across the lifespan. We identified
age-related changes in the contribution of parenchymal and CSF-like free water
to age-related changes in overall diffusivity that can be linked to changes in
tissue microstructure.
Introduction
The mean diffusivity (which is equal to 1/3 of the Trace of the diffusion tensor) of brain tissue water measured with a classical diffusion tensor MRI analysis, is known to change across the lifespan, decreasing in postnatal development and increasing in aging.1–4 With the availability of data acquired with more than one shell in the low b-value regime, it is possible to perform a biexponential analysis of the signal contribution of two non-exchanging water compartments: one assumed to be isotropic with diffusivity equal to the diffusivity of water at 37º C (CSF-like water), while the other is parenchymal water with lower diffusivity than the CSF-like water compartment and modeled using a full tensor.5 Using this model, it has been shown that in adult brain tissue the signal fraction of CSF-like water is about 20% of the total even in regions immune from CSF partial volume contaminatiom.5 In the current study, we applied this dual compartment DTI model to investigate CSF-like water and parenchymal diffusivity in childhood, early adulthood, and older adulthood, and examine how they change across the lifespan.Methods
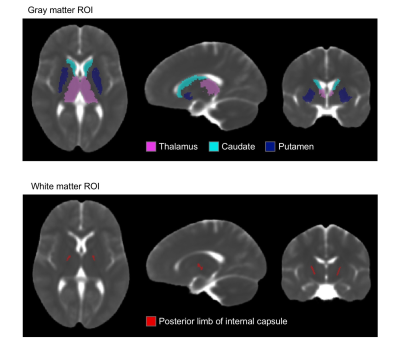
For our analysis, we used publicly available data. The pediatric sample included 142 subjects (age=0-22) with diffusion weighted images (DWIs) from the NIH MRI Normal Brain Development database (b=0, 300, 500, 800, 1100 s/mm2). The cohort of older subjects (age=56-91) included 59 ADNI3 cognitively normal participants (b=0, 500, 1000 s/mm2).6–8 TORTOISE was used for DWI preprocessing, nonlinear single compartment and dual compartment tensor fitting, and computing single compartment Trace (Trace-SC), dual compartment parenchymal Trace (Trace-P), and volume fraction of CSF-like water (VFCSF).9,10 We examined each metric in subcortical gray matter (thalamus, caudate, putamen) and white matter (posterior internal capsule) ROIs. These ROIs were selected as representative ROIs based on their anatomical location deep within the parenchyma to minimize CSF contamination (Figure 1). The analysis was performed only in voxels where the parenchymal volume fraction was at least 30% to further reduce potential CSF contamination. These ROIs were created on a study-specific template built using DRTAMAS.11,12 The white matter ROI was manually drawn on the study-specific template, and the subcortical gray matter ROIs from the Desikan atlas13 were warped from MNI space into study-specific template space using ANTs.14 ROIs were warped from template to native space, and mean values were computed in native space. We performed separate linear models for the following age groups: < 3, 3-22, and 56-91 years to examine age-related changes within each metric.Results
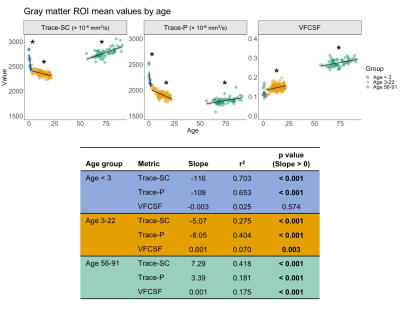
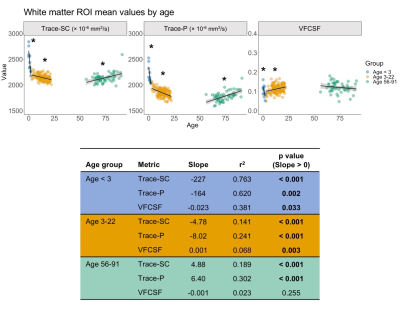
Using the dual compartment DTI model, we identified age-related changes in parenchymal and CSF-like water that contribute to changes in overall diffusivity. In early development, the dramatic decrease in diffusivity in gray matter is primarily driven by a decrease in parenchymal water with no contribution from changes in CSF-like water. As age increases into early adulthood, parenchymal water continues to decrease while CSF-like water increases, reducing overall diffusivity. In later adulthood, gray matter diffusivity increases due to increases in both the parenchymal and CSF-like water compartments. In white matter, decreased diffusivity in early development results from decreased water in both compartments, while childhood and early adulthood show the same pattern identified in the gray matter. In later adulthood, overall diffusivity increases but results from an increase in parenchymal water. Figures 2-3 show plots of mean values and linear model results.Discussion
Our investigation has shown that relative contributions of parenchymal and CSF-like water to overall diffusivity of the tissue vary throughout the lifespan. This is especially evident in gray matter, where the youngest and oldest groups share similarly high Trace-SC. Further examination of the parenchymal and CSF-like water compartments demonstrates that these high Trace-SC values originate from different water pools. In early development, high Trace-SC is driven by the parenchymal water, while in the older adult group, it is driven by increasing CSF-like water. Our results also indicate changes in the characteristics of tissue microstructure. The extracellular space contains water that is part of the parenchymal water compartment. In early brain development, the dramatic reduction in parenchymal water reflects the rapid decrease in extracellular space that occurs due to increasing cell density and myelination.1 CSF-like water can include contributions from various sources such as blood flow (Intra Voxel Incoherent Motion (IVIM)),15 excess water in perivascular spaces, and water in lacunae large enough (30 microns or more) to preclude fast exchange with tissue molecules. Therefore, an increase of CSF-like volume fraction in aging may be related to degenerative tissue changes as well as benign enlargement of perivascular spaces.Application of the dual compartment model allows for a better understanding of the contributions of parenchymal trace and CSF-like water to overall diffusivity throughout the lifespan. Our findings show that the two compartments contribute differently to overall diffusivity in development and aging and can be linked with changes in the microstructural characteristics of the tissue.
Acknowledgements
*Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
1. Yoshida, S., Oishi, K., Faria, A. V, and Mori, S. (2013). Diffusion tensor imaging of normal brain development. Pediatr. Radiol. 43, 15–27.
2. Pfefferbaum, A., and Sullivan, E. V. (2003). Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magn. Reson. Med. 49, 953–961.
3. Pfefferbaum, A., Adalsteinsson, E., Rohlfing, T., and Sullivan, E. V. (2010). Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiol. Aging 31, 482–493.
4. Salminen, L.E., Conturo, T.E., Laidlaw, D.H., Cabeen, R.P., Akbudak, E., Lane, E.M., Heaps, J.M., Bolzenius, J.D., Baker, L.M., Cooley, S., Scott, S., Cagle, L.M., Phillips, S., and Paul, R.H. (2016). Regional age differences in gray matter diffusivity among healthy older adults. Brain Imaging Behav. 10, 203–211.
5. Pierpaoli, C., and Jones, D.K. (2004). Removing CSF Contamination in Brain DT-MRIs by Using a Two-Compartment Tensor Model., in: Proceedings of the 12th Annual Meeting of ISMRM, Kyoto. pp. 1215.
6. Evans, A.C. (2006). The NIH MRI study of normal brain development. Neuroimage 30, 184–202.
7. Walker, L., Chang, L.C., Nayak, A., Irfanoglu, M.O., Botteron, K.N., McCracken, J., McKinstry, R.C., Rivkin, M.J., Wang, D.J., Rumsey, J., and Pierpaoli, C. (2016). The diffusion tensor imaging (DTI) component of the NIH MRI study of normal brain development (PedsDTI). Neuroimage 124, 1125–1130.
8. Zavaliangos-Petropulu, A., Nir, T.M., Thomopoulos, S.I., Reid, R.I., Bernstein, M.A., Borowski, B., Jack Jr., C.R., Weiner, M.W., Jahanshad, N., and Thompson, P.M. (2019). Diffusion MRI Indices and Their Relation to Cognitive Impairment in Brain Aging: The Updated Multi-protocol Approach in ADNI3. Front. Neuroinform. 13, 2.
9. Pierpaoli, C., Walker, L., Irfanoglu, M.O., Barnett, A., Basser, P.J., Chang, L.-C., Koay, C.G., Pajevic, S., Rohde, G.K., Sarlls, J.E., and Wu, M. (2010). TORTOISE: An Integrated Software Package for Processing of Diffusion MRI Data., in: Proceedings of the 19th Annual Meeting of ISMRM. Stockholm, Sweden, pp. 1597.
10. Irfanoglu, M.O., Nayak, A., Jenkins, J., and Pierpaoli, C. (2017). TORTOISEv3:Improvements and New Features of the NIH Diffusion MRI Processing Pipeline., in: Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, HI.
11. Irfanoglu, M.O., Nayak, A., Jenkins, J., Hutchinson, E.B., Sadeghi, N., Thomas, C.P., and Pierpaoli, C. (2016). DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage 132, 439–454.
12. Nayak, A., Sadeghi, N., Irfanoglu, M.O., and Pierpaoli, C. (2017). Diffusion-Tensor Based Morphometry (DTBM) of Normal Human Brain Development from Infancy to Adulthood., in: Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, HI, pp. 3371.
13. Desikan, R.S., Ségonne, F., Fischl, B., Quinn, B.T., Dickerson, B.C., Blacker, D., Buckner, R.L., Dale, A.M., Maguire, R.P., Hyman, B.T., Albert, M.S., and Killiany, R.J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest.
14. Avants, B.B., Tustison, N.J., Song, G., Cook, P.A., Klein, A., and Gee, J.C. (2010). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044.
15. Le Bihan, D., Breton, E., Lallemand, D., Aubin, M.L., Vignaud, J., and Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505.
Figures