0864
Fully-Automated Delineation of the Optic Radiation1The Australian e-Health Research Centre, CSIRO, Brisbane, Australia, 2Center of Neuroimmunology, Laboratory of Advanced Imaging in Neuroimmunological Diseases, Hospital Clinic Barcelona, Institut d'Investigacions Biomediques August Pi i Sunyer and Universitat de Barcelona, Barcelona, Spain, 3ITACA, Universitat Politècnia de València, Valencia, Spain
Synopsis
The optic radiation (OR), is often severed during temporal lobe resection, resulting in permanent quadrantanopia. To date all published tractography methods that delineate the OR require manual input (e.g. region-of-interest placement and adjustment), or appear to underestimate Meyer’s Loop, limiting their widespread clinical adoption. Here, we present and validate the CONSULT pipeline for OR delineation. This pipeline accepts unprocessed DICOM images as input and produces realistic subject-specific segmentations of the OR, including Meyer’s Loop, without need for any human input. Its validation in 183 datasets demonstrated plausible delineations that are in line with previous dissection studies.
Introduction
Meyer’s Loop of the optic radiation (OR), is often severed during temporal lobe resection, resulting in permanent quadrantanopia.1 Cadaver dissection studies have demonstrated that the distance between the temporal pole and most anterior point of Meyer’s loop (ML-TP distance) varies considerably between patients,2–6 and so this distance has been explored as a predictor of such surgically-induced partial blindness.1 Several groups have successfully delineated Meyer’s Loop using diffusion tractography7,8 but to date all published methods have required manual input (e.g. region-of-interest [ROI] placement and adjustment) from trained technicians, or appear to underestimate the ML-TP distance,7 limiting their widespread clinical adoption. The major sources of difficulty for delineating the OR via tractography include its twisting and branching morphology, as well as the apparent invisibility of the anatomically-correct seeding nucleus – the lateral geniculate nucleus (LGN) – in standard MR images. These factors lead to long processing times, manual placement of ROIs, and/or underestimated ML-TP distances,7 which clinically might lead to improper surgical planning and thus inadvertent severing of this tract during surgery. Here, we present and validate the Connectivity Based Neurosurgical Planning Tool (CONSULT) pipeline for delineation of the OR. This pipeline accepts unprocessed DICOM or NIfTI images as input and produces subject-specific segmentations of the OR, including Meyer’s Loop, without need for any human input.Methods
DatasetWe validated our CONSULT method using three datasets. The Barcelona dataset included 19 adults scanned at Hospital Clinic of Barcelona (T1: 0.9mm isotropic; diffusion MRI: 1.5mm isotropic, 30 x 1000s/mm2, 60 x 2000s/mm2, 90 x 3000s/mm2; standard fieldmap; total acquisition time 26min). The Human Connectome Project (HCP) dataset included 160 scans (preprocessed T1 and diffusion images). The Brisbane dataset included images of four adult neurosurgery patients acquired presurgically at the Herston Imaging Research Facility, Brisbane (T1: 1mm isotropic; diffusion MRI: 2mm isotropic; 20 x 1000s/mm2, 32 x 2000s/mm2, 60 x 3000s/mm2; total acquisition time 16min). The morphology of the OR was assessed qualitatively for all datasets. The ML-TP distance was measured from the tractography of the larger two datasets. All participants gave written informed consent and data acquisitions were approved by local ethics committees.
CONSULT Pipeline
CONSULT requires a T1 MPRAGE image and tractography-suitable diffusion MRI images. The T1 is preprocessed using N4ITK;9 skull stripped using HD-BET;10 and then denoised and tissue-segmented using Approximate Block Matching.11,12 Registration to both diffusion (rigid) and MNI152 space (non-linear) is performed using ANTs,13 in order to transform various ROIs defined in MNI space (see below) into native diffusion space. Diffusion image preprocessing includes denoising,14 removal of motion affected volumes, dewarping,15 and skull stripping. Fibre orientation dispersion maps are calculated using the Dhollander algorithm16 and multishell multitissue contrained spherical deconvolution.17
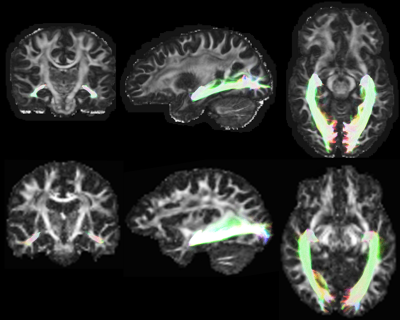
LGN delineation: To identify the LGN, the optic nerve and medial bundle of the OR are delineated using tractography seeded from the optic nerve near to the brainstem (Figure 1). V1 forms an inclusion mask. Several exclusion masks are applied, similar to those previously described.8 V1 and exclusion masks are identified by registration between diffusion and MNI space, in which these ROIs are predefined. The seeding zone of the optic nerve is identified using a convolutional neural network with U-Net18 like architecture, accepting T1 and FOD images. This network was originally trained using track density images (‘trackmaps’) of 500 HCP datasets (outside of the 160 processed with CONSULT) for which the optic nerve was delineated using tractography. Once tractography, seeded from the optic nerve, is complete, it is converted into approximation of the LGN by conversion into a binarised trackmap that is dilated and cropped to an approximate LGN mask (defined in MNI space).
Tractography is performed from the LGN, using several tractography-accelerating techniques, implemented in a forked version of MRtrix3. These included use of “ordered” inclusion ROIs; setting initial seeding directions on a voxelwise basis; and ‘retracking’ wherein streamlines that have failed (for example by hitting an exclusion ROI) are “rewound” to the nearest retracking ROI, a similar process to that normally performed in Anatomically Constrained Tractography.19
Results
The OR was successfully delineated in all 183 scans without error. Visual inspection suggested credible delineations in all cases (Figure 2). The median ML-TP distances were 25mm (min: 20.7; IQR: 23.8 – 26.7mm; max: 32mm) and 27mm (min: 12.4mm; IQR: 25.5 – 28.7mm; max: 32.5mm) for the Barcelona and HCP datasets, respectively, approximately matching the averages and ranges reported in dissection studies.2–6Discussion
The OR is a white matter tract with complex anatomy that is difficult to delineate, even with advanced neuroimaging. Although there are instances of successful delineations of this anatomy, all published pipelines require human input and/or may underestimate the anterior extent of Meyer’s Loop, which reduces their practicality in busy clinical settings. We have demonstrated a pipeline for delineating this anatomy that performs at least as well as known manual and semi-automatic pipelines, but is fully automated and thus requires no training for use. Qualitatively, this was able to delineate the OR in three datasets (183 scans), two of which were acquired on Hospital campuses in clinically acceptable timeframes. Quantitatively, the ML-TP distances delineated with this pipeline matched ranges seen in dissection studies, implying their suitability for clinical use.Acknowledgements
No acknowledgement found.References
1. Winston, G. P. Epilepsy surgery, vision, and driving: what has surgery taught us and could modern imaging reduce the risk of visual deficits? Epilepsia 54, 1877–88 (2013).
2. Ebeling, U. & Reulen, H. J. Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir. (Wien). 92, 29–36 (1988).
3. Peuskens, D. et al. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery 55, 1174–1183 (2004).
4. Rubino, P. A., Rhoton, A. L., Tong, X. & De Oliveira, E. Three-dimensional relationships of the optic radiation. Neurosurgery 57, 219–227 (2005).
5. Choi, C., Rubino, P. A., Fernandez-Miranda, J. C., Abe, H. & Rhoton, A. L. Meyer’s loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery 59, ONS228-35; discussion ONS235-6 (2006).
6. Chowdhury, F. H. & Khan, A. H. Anterior & lateral extension of optic radiation & safety of amygdalohippocampectomy through middle temporal gyrus: a cadaveric study of 11 cerebral hemispheres. Asian J. Neurosurg. 5, 78–82 (2010).
7. Chamberland, M., Tax, C. M. W. & Jones, D. K. Meyer’s loop tractography for image-guided surgery depends on imaging protocol and hardware. NeuroImage Clin. 20, 458–465 (2018).
8. Martínez-Heras, E. et al. Improved Framework for Tractography Reconstruction of the Optic Radiation. PLoS One 10, e0137064 (2015).
9. Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–20 (2010).
10. Isensee, F. et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum. Brain Mapp. 40, 4952–4964 (2019).
11. Reid, L. B. & Pagnozzi, A. M. Rapid Training Data Generation for Tissue Segmentation Using Global Approximate Block-Matching with Self-organizing Maps. in Lecture Notes in Computer Science (eds. Stoyanov, D. et al.) 11045, 110–118 (Springer International Publishing, 2018).
12. Reid, L. B., Gillman, A., Pagnozzi, A. M., Manjón, J. V. & Fripp, J. MRI Denoising and Artefact Removal Using Self-Organizing Maps for Fast Global Block-Matching. in Lecture Notes in Computer Science (eds. Bai, W. et al.) 11075, 20–27 (2018).
13. Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
14. Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406 (2016).
15. Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2015).
16. Dhollander, T., Raffelt, D. & Connelly, A. Accuracy of response function estimation algorithms for 3-tissue spherical deconvolution of diverse quality diffusion MRI data. in 26th International Society of Magnetic Resonance in Medicine 26, 1569 (2018).
17. Jeurissen, B., Tournier, J.-D., Dhollander, T., Connelly, A. & Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103, 411–426 (2014).
18. Ronneberger, O., Fischer, P. & Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. in Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2015 (eds. Navab, N., Hornegger, J., Wells, W. M. & Frangi, A. F.) 234–241 (Springer International Publishing, 2015).
19. Smith, R. E., Tournier, J.-D., Calamante, F. & Connelly, A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62, 1924–1938 (2012).
Figures