0853
Myelin weighted tractography: a new way to investigate bundles myelination in-vivo?
Simona Schiavi1,2, Po-Jui Lu3,4, Matthias Weigel3,4,5, Derek K. Jones6,7,8, Ludwig Kappos3,4, Cristina Granziera3,4, and Alessandro Daducci1
1Department of Computer Science, University of Verona, Verona, Italy, 2DINOGMI, University of Genoa, Genoa, Italy, 3Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 4Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 5Radiological Physics, Department of Radiology, University Hospital Basel and University of Basel, Basel, Switzerland, 6Cardiff University Brain Research Imaging Centre, Cardiff University, Cardiff, United Kingdom, 7Neuroscience and Mental Health Research Institute, Cardiff University, Cardiff, United Kingdom, 8Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia
1Department of Computer Science, University of Verona, Verona, Italy, 2DINOGMI, University of Genoa, Genoa, Italy, 3Departments of Medicine, Clinical Research and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 4Translational Imaging in Neurology (ThINk) Basel, Department of Medicine and Biomedical Engineering, University Hospital Basel and University of Basel, Basel, Switzerland, 5Radiological Physics, Department of Radiology, University Hospital Basel and University of Basel, Basel, Switzerland, 6Cardiff University Brain Research Imaging Centre, Cardiff University, Cardiff, United Kingdom, 7Neuroscience and Mental Health Research Institute, Cardiff University, Cardiff, United Kingdom, 8Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia
Synopsis
Tractometry is a widely used tool to investigate microstructural properties that reflect differences in white matter tracts. It consists in averaging tissue features (obtained from any voxel-wise map) along streamlines recovered with diffusion tractography. To overcome the bias introduced by the average, we propose a new method to deconvolve on each individual tract the actual value measured by the microstructural map. For the first time we were able to assess the bundle-specific myelin content by combining tractography with two myelin-sensitive sequences and we obtained good agreement with previously reported values on the cortex.
Introduction
Voxel-wise myelin maps of brain white matter (WM) derived from MR sequences tend to be spatially flat with no apparent spatial pattern structure (Figure 1), therefore they are mostly used to study pathological tissues or to perform average measures along major tracts1,2. Very recently, Baumeister et al. attempted to investigate average myelin values in sections along fiber bundles rather than integrating over the whole tracts3. Although such methods provide useful macroscopic information on the quantity of myelin in different fiber bundles2,3, we argue that considering the mean of a microstructural map along a WM tract and/or investigating values that derive from a voxel average of more than one bundle provide biased results. Here, we propose a novel technique that is able to decouple the actual myelin content of each individual bundle by performing a deconvolution in the space of fiber tracts.Methods
On four healthy volunteers, using a Siemens Prisma 3T scanner, we acquired a 3D-MP2RAGE with $$$1mm^3$$$ isotropic spatial resolution, a myelin water imaging as in 4 with resolution $$$1x1x5mm^3$$$, a Magnetization Transfer image with saturation (MTsat) with resolution $$$1.3mm^3$$$ isotropic, and diffusion weighted images (DWI) with a total of 107 directions subdivided in 5 shells with maximum b-value$$$=3000s/mm^2$$$ and resolution $$$1.8mm^3$$$ isotropic. DWIs were pre-processed to remove noise, eddy currents and motion distortions. They were then upsampled and registered to MP2RAGE to perform anatomically constrained multi-shell multi-tissue tractography with a deterministic algorithm using MRtrix3. We constructed a whole brain tractogram using 3M streamlines. Myelin water fraction (MWF) and myelin volume fraction (MVF) maps were fitted from myelin water and MTsat images as in 4,5. Myelin maps were also co-registered to MP2RAGE space. We then modified the Convex Optimization Modeling for Microstructure Informed Tractography (COMMIT6) in order to perform the deconvolution procedure using these maps. This allowed us to filter out redundant and implausible streamlines as in the original framework while, at the same time, decouple the myelin content of each one of them. The remaining streamlines (i.e. those with contribution $$$>0$$$ according to COMMIT) were grouped in bundles depending on the pair of cortical regions they connected. To define the regions we segmented the MP2RAGE images with FreeSurfer using the standard Desikan-Killiany atlas7. Finally, to estimate the myelin content of a bundle, we summed the contributions assigned by COMMIT to the streamlines belonging to that bundle.We compared our proposed method to the classical tractometry approach as it is widely used in the field, which averages the contribution of each streamline obtained from the mean of the myelin map along its path.
Results and Discussion
We decided to employ a deterministic tracking algorithm to avoid the presence of a high number of false positive connections that could bias the results. Figure 2 reports the results obtained projecting on the cortex the individual streamline values recovered applying COMMIT or tractometry on MWF or MVF maps and dividing by the cortical region volumes to avoid biases due to the regions extension. We can see that COMMIT values are in very good agreement with what reported in previous histological and imaging studies8, which show higher myelin content in primary motor, sensory, visual and auditory areas in comparison to other cortical brain regions9. High levels of myelination in the basal temporal regions are also evident, as previously reported in myeloarchitectonic studies9. Results obtained with tractometry are on the other hand not really corresponding to the current histological knowledge about cortical myelination characteristics: in fact, this approach recovers higher values in the frontal lobes than in the primary motor cortex. Figure 3 reports the reproducibility of the results obtained with our approach on all the enrolled subjects using MWF maps. The results hold also for MVF.Figure 4 shows an orthogonal view of the streamlines color-coded with the myelin content of the bundle they are in. While tractometry maps are rather flat for both techniques, with the COMMIT approach we can distinguish between different bundles. This gives us the unique opportunity of investigating individual bundle characteristics useful to understand functional properties as conduction delay.
Finally, in Figure 5 we show only few bundles which belong to the left motor network. Again, in agreement with the literature, we found that the myelin content associated by COMMIT results in higher myelination of the bundles connecting the left and right precentral gyri (PrCG) and PrCG with the medulla than those that connect the PrCG with the subcortical nuclei10. We also show the highest myelinated bundle recovered from COMMIT that connect the two hemispheres which coincides with the anterior frontal bundle, as already reported in 11.
Conclusions
For the first time we were able to assess the actual bundles myelin content by applying COMMIT on myelin maps. This allows us to investigate the bundle-specific myelination content, not a biased average of this quantity. The proof of concept presented in this work opens to the possibilities of in-vivo investigations of neuronal pathways that, to date, were possible only post-mortem.Acknowledgements
This work was supported by the Rita Levi Montalcini Programme of the Italian Ministry of Education, University and Research (MIUR). This work was funded by the Swiss National Funds PZ00P3_154508, PZ00P3_131914 and PP00P3_176984.References
- I. Lipp, D. K. Jones, S. Bells, E. Sgarlata, C. Foster, R. Stickland, A. E. Davidson, E.C. Tallantyre, N.P. Robertson, R.G. Wise, V. Tomassini. Comparing MRI metrics to quantify white matter microstructural damage in multiple sclerosis. Human Brain Mapping. 2019, 40(10):2917-2932.
- J.D. Yeatman, B.A. Wandell, A.A. Mezer. Lifespan maturation and degeneration of human brain white matter. Nature Communication. 2014 5:4932.
- T.R. Baumeister, S.H. Kolind, A.L. MacKay, M.J. McKeown. Inherent spatial structure in myelin water fraction maps. Magnetic Resonance Imaging. 2019.
- TD Nguyen, K Deh, E Monacan, et al. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med. 2016; 76(2):456-65.
- S. Mohammadi, D. Carey, F. Dick, J. Diedrichsen, M.I. Sereno, M. Reisert, M.F. Callaghan, N. Weisskopf. Whole-Brain In-vivo Measurements of the Axonal G-Ratio in a Group of 37 Healthy Volunteers. Frontiers in Neuroscience. 2015, 9.
- A. Daducci, A. Dal Palù, A. Lemkaddem, J.P. Thiran. COMMIT: Convex Optimization Modeling for Microstructure Informed Tractography, IEEE Trans. Med. Imaging., 2014, 33:246-57.
- R.S. Desikan, F. Segonne, B. Fiscal B.T. Quinn, B.C. Dickerson, D. Blacker, R.L. Buckner, A.M. Dale, R.P. Maguire, B.T. Hyman, M.S. Albert, R.J. Killiany. An automated labeling system for subdividing the human cerebralcortex on MRI scansintogyralbasedregions of interest. Neuroimage 2006, 31:968-980.
- J.P. Marques, D.Khabipova, R. Gruetter. Studying cyto and myeloarchitecture of the human cortex at ultra-high field with quantitative imaging: R1, R2* and magnetic susceptibility. NeuroImage 2017.
- R. Nieuwenhuys, C.A.J. Broere. A map of the human neocortex showing the estimated overall myelin content of the individual architectonic areas based on the studies of Adolf Hopf. Brain Struct Funct. 2017, 222: 465.
- F. Kimura, C. Itami. Myelination and isochronicity in neural networks. Front. Neuroanat. 2009, 3:12.
- N. Stikov, L.M. Perry, A. Mezer, E. Rykhlevskaia, B.A. Wandell, J.M. Pauly, R.F. Dougherty. Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. NeuroImage, 54:1112-1121, 2011.
Figures
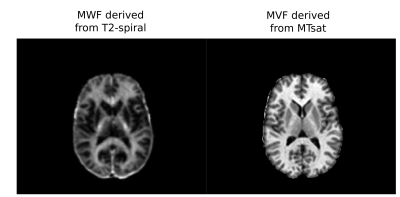
Figure 1: Axial view of MWF map obtained from the T2-spiral acquisition and MVF map obtained from MTsat acquisition for a representative subject in our study.
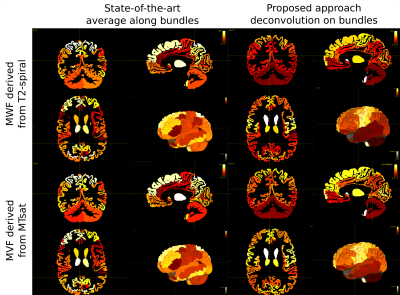
Figure 2: Comparison of the results obtained with state-of-the-art tractometry, computing the average of microstructural properties along the streamlines, and the proposed approach, which instead decomposes the myelin content on individual components associated to each streamline. The images were obtained by projecting on the cortex the individual streamline values recovered applying COMMIT or tractometry on MWF or MVF maps and dividing by the cortical region volumes to avoid biases due to the region extension.
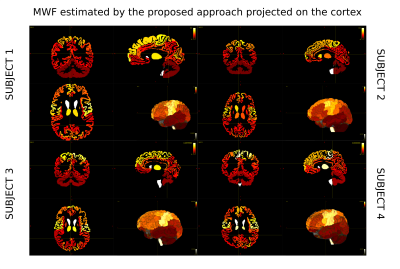
Figure 3: Reproducibility of the proposed method across 4 different healthy subjects. Figures show the projection on the cortex of the values obtained by applying COMMIT on MWF maps of each subject.
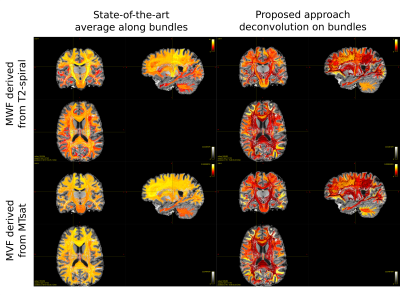
Figure 4: Comparison of the results obtained with state-of-the-art tractometry, computing the average of microstructural properties along the streamlines, and the proposed approach, which instead decomposes the myelin content on individual components associated to each streamline. The images were obtained by color-coding the streamlines with the myelin content of the bundle they are in, estimated applying COMMIT or tractometry on MWF or MVF maps.

Figure 5: Example of different myelinated bundles recovered by applying COMMIT on MWF or MVF map. We show few bundles that are part of the left motor network. In agreement with the literature, myelin content associated by COMMIT results in higher myelination of the bundles connecting the left and right precentral gyri (PrCG) and PrCG with the medulla than those that connect the PrCG with the subcortical nuclei.