0848
Diffusion MRI Atlases from the Human Connectome Project Data1QMI, NIBIB/NIH, Bethesda, MD, United States, 2The Henry Jackson Foundation for the Advancement of Military Medicine, Rockville, MD, United States
Synopsis
In this work, we have created diffusion MRI (dMRI) atlases from the young adult Human Connectome Project (HCP) data. In order to achieve increased anatomical detail and to enable subsequent morphological analysis, we have reprocessed the entire HCP1200 dataset. The DTI atlases, derived scalar maps and JHU atlas inspired white matter ROIs have been made publicly available. The reprocessed DWIs will be made available for HARDI analysis in the near future.
Introduction
The young adult Human Connectome Project1 (HCP) provides one of the largest diffusion MRI (dMRI) dataset acquired with very high resolution. This dataset has the potential to provide insights into understanding "the human brain" and can also be used as a normative control database for understanding other brain diseases. For such a purpose, all the subjects in the database need to be registered to a common template space, where a population representative atlas and the deformations that map each subject to this atlas are created. To our knowledge, the work from Yeh et al.2 is the only attempt to provide such an atlas, with a specific focus on connectivity. In this work, our goal was to provide such an atlas for both DTI3 and HARDI models.Methods
Dataset: In HCP1200 dataset 1021 subjects have dMRI scans. Fifty of these scans were found to contain either atypical anatomy or uncorrectable distortion artifacts due to non-diffeomorphic leaking and were excluded from processing, resulting in 971 subjects (520 females, 451 males).Processing: The unprocessed HCP dataset was acquired with a very high resolution at the expense of severe EPI distortions. These distortions were corrected with post-processing, however, for several subjects, residual distortion effects are still present in the corrected data. For tractography, such minor distortions can affect connectivity negatively4. Additionally, if the final dMRI atlas and subject specific deformation fields are to be used for morphometric analysis, the DWIs need to be near-perfectly aligned to structural images, with correct anatomy. For these reasons, the entire HCP unprocessed dataset was reprocessed with special settings with the TORTOISE5 dMRI processing pipeline.
For each subject, voxelwise B-matrices due to gradient nonlinearities were initially generated at the subject's native space. Subsequently, denoising and Gibbs ringing removal was performed on raw DWIs. Motion&eddy-currents distortion correction was performed while reorienting the voxelwise B-matrices and sampling the correct position in the scanner due to motion. Susceptibility distortion correction was performed by DRBUDDI6 with gradient nonlinearity fields as initial tranforms, which also combined the RL and LR encoded data into a single one. The final output of DRBUDDI was in the space of HCP provided T2W structural image at 1mm isotropic resolution.
Atlas creation: The diffusion tensor based atlas creation tool, DRTAMAS7, was used to create the final DTI atlas. DRTAMAS favors the deviatoric part of the diffusion tensor in regions where anisotropy is high and the mean diffusivity and T1W/T2W information where it is low, to achieve good alignment in both white matter and cortical gray matter regions. In this study, three atlases were created: 1) female, 2) male and 3) population dMRI atlas. For each case, DRTAMAS also generated T1W and T2W templates. The deformations generated for each subject were subsequently applied to diffusion weighted images to transform them onto the template space for subsequent HARDI analysis. Corresponding voxelwise b-matrices were updated based on these deformation fields. Several tensor-tensor derived maps were computed and made available.
ROIs: An experienced image analyst drew 48 white matter ROIs on the population template. These ROIs were inspired by the JHU atlas ROIs, however, they were centered along the fiber bundles, hence slightly smaller.
Results
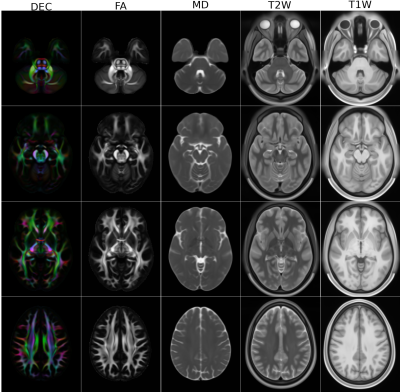
Figure 1 displays the DEC maps for the first three subjects in HCP, processed with TORTOISE and HCP pipelines. Because of the improvements in denoising, susceptibility induced distortion correction and higher resolution resampling brought by TORTOISE, its generated images have improved anatomical accuracy within the brainstem, especially for corticospinal tracts, and more anatomical details in temporal lobes, which makes them morphologically more suitable for dMRI-based atlas creation.Figure 2 displays the DEC, FA, MD, T2W and T1W images for the male atlas at different slice levels. Even though a very large number of subjects were used for the creation of the templates, the anatomical details of all the atlases are of high quality.
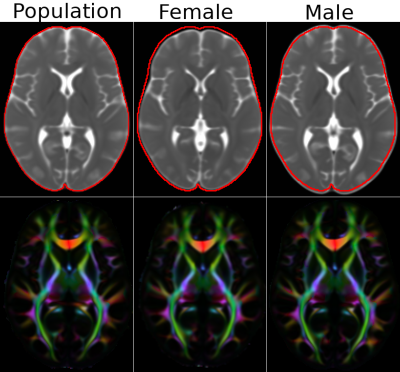
Figure 3 displays the MD and DEC maps for the population, male and female atlases. The images are visualized at the same slice level, not anatomical locations, which are different due to size differences. To indicate this size difference, a contour was drawn at the periphery of the population template and transferred to the other two.
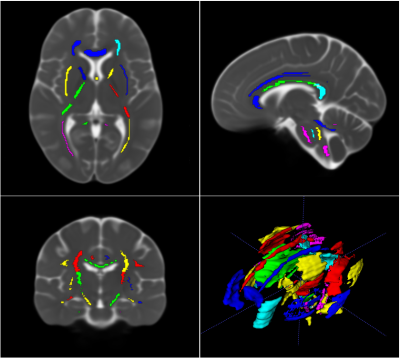
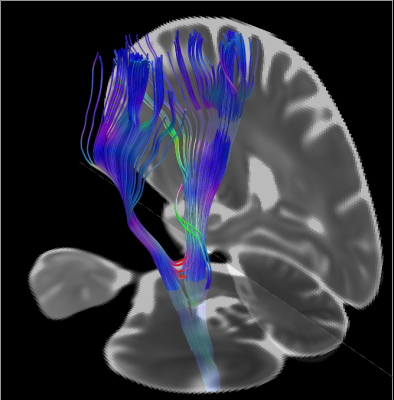
Figure 4 displays the ROIs overlayed on the MD map of the population template and Figure 5 displays the DTI tractography output from the left and right corticospinal tract ROIs.
Data availability: All three DTI atlases, their corresponding derived maps and the ROIs are currently available to download to at https://tortoise.nibib.nih.gov. Our intention is also to provide the TORTOISE processed DWIs and their corresponding voxelwise B-matrices both in their structural image space and their template space, once the logistics for data storage and access is clarified and necessary data sharing permissions are obtained.
Conclusions
In this work, we have reprocessed the HCP1200 diffusion MRI dataset and created three population atlases that can be used for morphometric analysis, behavioral correlation studies or even as a normative healthy control template for studies focusing on brain disease analysis. The templates, derived scalar maps and the white matter ROIs are already publicly available with the reprocessed DWI data to be released in the future.Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering in the National Institutes of Health. The contents of this work do not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.References
1. Van Essen, D.C., Ugurbil, K., Auerbach, E., Barch, D., Behrens, T.E., Bucholz, R., Chang, A., Chen, L., Corbetta, M., Curtiss, S.W., Della Penna, S., Feinberg, D., Glasser, M.F., Harel, N., Heath, A.C., Larson-Prior, L., Marcus, D., Michalareas, G., Moeller, S., Oostenveld, R., Petersen, S.E., Prior, F., Schlaggar, B.L., Smith, S.M., Snyder, A.Z., Xu, J., Yacoub, E., 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222–2231.
2. Fang-Cheng Yeh, Sandip Panesar, David Fernandes, Antonio Meola, Masanori Yoshino, Juan C. Fernandez-Miranda, Jean M. Vettel, Timothy Verstynen,"Population-averaged atlas of the macroscale human structural connectome and its network topology", NeuroImage,178,2018,Pages 57-68,
3. Basser PJ, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal ofMagnetic Resonance 1994;103:247–254.
4. Irfanoglu MO, Walker L, Sarlls J, Marenco S, Pierpaoli C. Effects of image distortions originating from susceptibilityvariations and concomitant fields on diffusion MRI tractography results. Neuroimage 2012;15(61):275–288.
5. Pierpaoli C, Walker L, Irfanoglu MO, Barnett AS, Chang LC, Koay CG, et al. TORTOISE: An integrated software packagefor processing of diffusion MRI data. In: Proceedings of International Society of Magnetic Resonance in Medicine; 2010.p. 1597.
6. Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C. DR-BUDDI: (Diffeomorphic Registration for Blip-Upblip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage 2015;106:284–289.
7. Irfanoglu MO, Nayak A, Jenkins J, Hutchinson EB, Sadeghi N, Thomas CP, et al. DR-TAMAS: Diffeomorphic Registrationfor Tensor Accurate Alignment of Anatomical Structures. NeuroImage 2016;132:439 – 454.
Figures
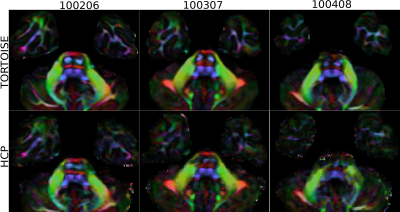
Figure 1. TORTOISE and HCP processing of three subjects displayed at the level of the temporal lobes and brainstem. The anatomy in the brainstem, specifically corticospinal tracts, is more accurate with TORTOISE processing. Additionally, the improved susceptibility distortion correction, denoising and resampling to higher resolution provides more detailed anatomy in the temporal lobes, which makes TORTOISE processing more suitable for atlas creation.