0844
Unprecedented diffusion weighting and exchange resolution of cellular and sub-cellular structures in live and fixed neural tissue1Eunice Kennedy Shriver National Institutes of Child Health and Human Development, National Institutes of Health, Bethesda, MD, United States, 2National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 3National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 4College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China
Synopsis
Diffusion and exchange methods are developed using the large static gradient produced by a single-sided permanent magnet and provide resolution to water within sub-micron membrane structures. Using tissue delipidation methods, we show that water diffusion is restricted solely by lipid membranes. Most of the diffusion signal can be assigned to water in tissue which is far from membranes. The remaining 25% can be assigned to water restricted within membrane structures at the cellular, organelle, and vesicle levels. Diffusion exchange spectroscopy measures water exchanging between membrane structures and free environments at 100 s−1.
Introduction
In systems with microscopic fluid compartments which communicate on timescales similar to the diffusion encoding time, the resolution of pulsed gradient spin echo (PGSE) NMR is limited by the maximum gradient strength and the gradient pulse duration. Spin echoes acquired in a large static magnetic field gradient (SGSE) can break this microstructural resolution limit and probe nanoscale structures in sub-millisecond timescales.1 Under a static gradient , the signal attenuation regime is determined by the restriction length $$$l_s$$$ relative to the free diffusion length $$$l_D=\sqrt{D_0 \tau}$$$ and the dephasing length $$$l_g=(D_0/\gamma g)^{1/3}$$$.2 Unlike the Stejskal-Tanner PGSE experiment,3 in which $$$g$$$ and thus $$$l_g$$$ is incremented while the diffusion encoding time ($$$\Delta$$$ for PGSE, $$$\tau$$$ for SGSE) and thus $$$l_D$$$ is held constant, the Carr-Purcell SGSE experiment4 increments $$$\tau$$$ and thus $$$l_D$$$ while the static gradient holds $$$l_g$$$ constant. Both methods encode mobility through signal attenuation. One benefit of the SGSE is that free water signal is efficiently attenuated (the shortest $$$\tau$$$ for a given $$$g$$$), whereas signal from water which is restricted persists, allowing for the identification of restricted and free water at very short time and length scales. With a 15.3 T/m gradient and $$$D_0=2.15 \times 10^{-9}$$$ m2/s for water at 25°C, freely diffusing water has significantly attenuated by $$$\tau>0.3$$$ ms ($$$l_d>l_g=800$$$nm) and the remaining signal is mostly made up of water for which diffusion is impeded on that time and length scale. Diffusion exchange spectroscopy (DEXSY) encodes the mobility of spins at two instances separated by a mixing time.5 The rate that spins exchange between regions of differing mobility can be quantified by varying the mixing time. DEXSY-based methods can measure the permeability of cell membranes to water.6 New methods to clear lipid membranes from tissue provide a means to interrogate the effects of membranes on water mobility.7Methods
NMR measurements were performed at 13.79 MHz and $$$g$$$=15.3 T/m on a low-cost, single-sided permanent magnet known as the PM-10 NMR MOUSE with a Kea2 spectrometer (Magritek).8,9 A solenoid RF coil and circuit board were built in-house to maximize filling factor and SNR. Experiments were performed on isolated spinal cords of neonatal Swiss Webster wild type mice, kept alive (n=9) or fixed in 4% paraformaldehyde (n=6). Additional experiments were performed on fixed spinal cords after clearing lipids with Triton X surfactant (n=2). Static gradient spin echo (SGSE) 1-D diffusion10 (43 points, $$$\tau=0.05$$$ to 6.55ms, $$$b=$$$ 0.14 to 3,200,000 $$$\mathrm{s/mm^2}$$$) and 2-D DEXSY5 ( 21 $$$\times$$$ 21 points, $$$\tau_1,\tau_2=$$$0.2 to 3.3ms) experiments with CPMG acquisition (2000 echoes, TE=25μs, 400μm slice, TR=2s) were used to probe water mobility and exchange between more and less mobile environments. A rapid 4-point DEXSY method (See Ref. [11] for details) was used to measure diffusion-weighted average exchange fractions and rates with high temporal resolution.Results and Discussion
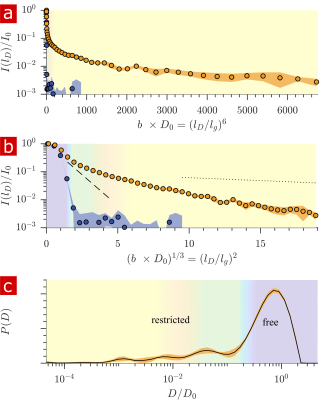
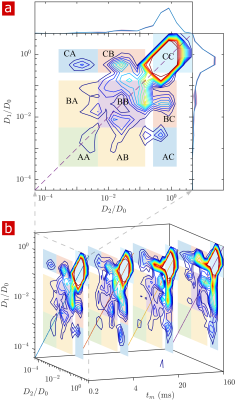
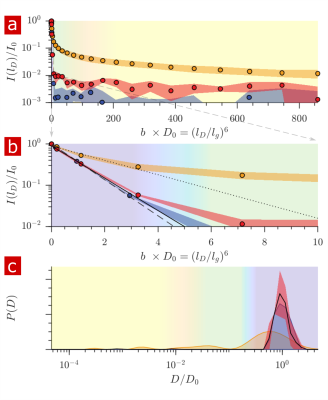
Fig. 1 shows the signal attenuation and distribution from SGSE experiments performed on a fixed spinal cord. The broad distribution (Fig. 1c) shows about 25% of the signal arising from restricted water pools. Signal attenuation models of water localized near surfaces2 and restricted in spherical pores8 indicate that the experimental system measures water within sub-micron restrictions, consistent with membrane structures at the cellular, organelle, and vesicle level. Water exchanges between restricted and free environments with a rate of roughly 100s-1 (n=6), as measured from both the full DEXSY (Fig. 2) and the rapid DEXSY methods. This rate is significantly faster than results of previous studies of water exchange in neural tissue, likely due to the system being sensitive to smaller structures with higher surface to volume ratios and to structures with larger permeability. Clearing lipids with 10% wt. Triton X surfactant shows membranes to be the origin of 99% of the water restriction (Fig. 3).Conclusion
Large static gradients provide a means to reach unprecedented diffusion weighting and probe water within sub-micron membrane structures. These components may include water within cells, cell processes, and organelles which could never before be isolated. DEXSY-based methods combined with large static gradients may provide measures of permeability of sub-cellular structures. Exchange rates indicate that standard PGSE methods cannot reach such high resolutions due to fluid moving between compartments during encoding. The sensitivity of diffusion NMR to tissue microstructure is almost entirely due to membranes.Acknowledgements
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke.References
1. R Kimmich, W Unrath, G Schnur, and E Rommel. NMR measurement of small self-diffusion coefficients in the fringe field of superconducting magnets. Journal of Magnetic Resonance, 91(1):136–140, 1991.
2. MD Hürlimann, KG Helmer, TM deSwiet, and PN Sen. Spin echoes in a constant gradient and in the presence of simple restriction. Journal of Magnetic Resonance, 113:260–264, 1995.
3. EO Stejskal and JE Tanner. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. The journal of chemical physics, 42(1):288–292, 1965.
4. HY Carr and EM Purcell. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Physical review, 94(3):630, 1954.
5. P. T. Callaghan and I. Furó. Diffusion-diffusion correlation and exchange as a signature for local order and dynamics. The Journal of Chemical Physics, 120(8):4032–4038, 2004.
6. I Åslund, A Nowacka, M Nilsson, and D Topgaard. Filter-exchange PGSE NMR determination of cell membrane permeability. Journal of Magnetic Resonance, 200(2):291 – 295, 2009.
7. C Leuze, M Aswendt, E Ferenczi, et al. The separate effects of lipids and proteins on brain MRI contrast revealed through tissue clearing. Neuroimage, 156:412–422, 2017.
8. G Eidmann, R Savelsberg, P Blümler, and B Blümich. The NMR mouse, a mobile universal surface explorer. Journal of Magnetic Resonance, 122:104–109, 1996.
9. R Bai, A Klaus, T Bellay, C Stewart, S Pajevic, U Nevo, H Merkle, D Plenz, and P J Basser. Simultaneous calcium fluorescence imaging and MR of ex vivo organotypic cortical cultures: a new test bed for functional MRI. NMR in Biomedicine, 28(12):1726–1738, 2015.
10. D.G. Rata, F. Casanova, J. Perlo, et al. Self-diffusion measurements by a mobile single-sided NMR sensor with improved magnetic field gradient. Journal of Magnetic Resonance, 180(2):229 – 235, 2006.
11. TX Cai, D Benjamini, ME Komlosh, et al. Rapid detection of the presence of diffusion exchange. Journal of Magnetic Resonance, 297:17–22, 2018.
Figures