0840
Ex Vivo MRI for Direct Radiologic-Histologic Correlation in the Pancreas: Protocol Development with Cadaver Specimens1Department of Radiology, University of Wisconsin, Madison, WI, United States, 2Department of Pathology, University of Wisconsin, Madison, WI, United States, 3Department of Surgery, University of Wisconsin, Madison, WI, United States, 4Department of Surgery, Georgetown University, Washington DC, MD, United States, 5Department of Radiology and Department of Medical Physics, University of Wisconsin, Madison, WI, United States
Synopsis
The purpose of this study was to develop a protocol for use of a previously validated radiologic-histologic correlation device to evaluate the pancreas with ex vivo MRI. Precise radiologic-histologic correlation of pancreatic anatomy was achieved in cadaveric pancreas specimens. The final protocol will be applied to co-localize pancreas cancer margins radiologically and histologically, as well as nodal burden in pancreaticoduodenectomy specimens.
Introduction
Discrepancies between radiologic and pathologic tumor margins, as well as nodal burden in pancreas cancer are a major challenge that have important prognostic implications in the treatment of pancreas cancer [1-4]. The discrepancies are secondary to the challenges of in-vivo pancreas imaging and the complexity of pathologic processing [5-7]. We aim to improve our understanding of these discrepancies via high resolution ex vivo MRI of pancreas specimens using an MRI compatible radiologic-histologic correlation device (RHCD, Figure 1). The purpose of this work was to develop a protocol to co-localize radiologic and histologic anatomy of human cadaver pancreas specimens using ex vivo MRI and an RHCD. This study serves as a prelude to application of the RHCD to correlate histologic and radiologic pancreas cancer margins and nodal burden in resected surgical specimens and to quantify neoadjuvant treatment response and the extent of vascular invasion of pancreas cancer.Methods
SpecimensIn this prospective study, pancreas specimens were obtained from patients undergoing autopsies for non-traumatic and non-surgical deaths. Per existing institutional pathology protocol, the specimens were sectioned longitudinally and fixed in formalin for 24 hours prior to any further processing or imaging.
Radiologic-histologic correlation device (RHCD)
Our group previously developed and validated an MR-compatible RHCD for co-localizing liver lesions in porcine specimens [8] as well as small liver lesions in patients who underwent liver resection for liver metastases from a primary colorectal cancer [9]. These studies demonstrated excellent radiologic-histologic correlation of tumor location and anatomy.
Briefly, the RHCD is a Plexiglas 27x14x14cm3 MRI-compatible device with 1cm grid patterns in 3 dimensions, made visible on MR by silicone gel infilling, and sectioning planes in the x-axis orientation (Figure 1). After formalin fixation, specimens were placed in a casting material (alginate) within the RHCD (Figure 1) to maintain an axial orientation relative to the x-axis (sectioning axis).
MR Protocol
All specimens were then imaged using a clinical 3T MRI system (GE Signa PET/MR, GE Healthcare, Waukesha WI) using a high-resolution (0.8mm isotropic) fat suppressed 3D T1-weighted fast spoiled gradient echo (T1w-SGRE), a 3D fat suppressed T2-weighted fast-spin-echo (T2w-FSE), and a commercial chemical shift encoded-MRI fat quantification method (IDEAL-IQ, GE Healthcare, Waukesha, WI). MR images were acquired using a single channel quadrature head coil. Acquisition parameters for T1w 3D-SGRE scan included: TR=12.3ms, TE=1.7ms, RBW=+/-62.5kHz, FOV=14.4x22.4x32cm, Matrix size=180x280x416, spatial resolution=0.8x0.8x0.8mm3, flip angle=20, and scan time=10:19. Acquisition parameters for T2w 3D-FSE included: TR=3.3s, TE=100ms, ETL=130, RBW=+/-62.5kHz, FOV=14.4x22.4x32cm, Matrix size=180x280x416, spatial resolution=0.8x0.8x0.8mm3 and scan time=16:10. Acquisition parameters for IDEAL-IQ included: TR=9.3ms, TE=1.3, 2.3, 3.2, 4.1, 5.0, 5.9ms, RBW=+/-62.50kHz, FOV=14.4x19.2x32cm, Matrix size=144x192x320, spatial resolution=1x1x1mm3, flip angle=4, and scan time=13:08.
Pathologic Processing and Analysis
After imaging, specimens were sectioned axially via the x-axis sectioning planes. Specific areas of interest were localized using the RHCD grids. The specimen was then removed from the alginate, and processed in accordance with institutional tissue-specific pathology protocols. The final combined radiology-pathology protocol was approved by a team of attending pathologists, hepatobiliary surgeons, and abdominal MRI radiologists. Summary statistical analysis was conducted with SAS.
Results
Six cadaver pancreas specimens were obtained (4 total pancreatectomies, 2 partial pancreatectomies) and underwent the final combined MR and pathology protocols. There was excellent identification and measurement of all structures of interest, including surgically relevant margins, bile and pancreatic duct dimensions, vein dimensions, and nodal burden (Table 1, Figures 1-4). Additionally, proton density fat fraction (PDFF) was measured for each anatomic region of the pancreas (Table 1). Direct radiologic-histologic correlation provided detailed information regarding the histologic details of radiologic points of interest (Figure 2).Discussion
Radiologic histologic correlation using ex vivo MRI in pancreas specimens is highly feasible. This technology conveys precise anatomic relationships between the pancreas and adjacent structures that are not visible with in vivo imaging. With successful creation of an ex vivo imaging protocol, we will next apply this approach to the evaluation of pancreas cancer margins and nodal burden in pancreaticoduodenectomy specimens.Consclusion
Given the high resolution achieved with this protocol, we anticipate precise co-localization of radiologic and histologic structures and relationships of clinical interest in pancreas cancer specimens. This will include pancreas cancer margins, nodal disease-burden, tumor response to neoadjuvant therapy, and extent and location of vascular involvement.Acknowledgements
The authors would like to acknowledge and thank the University of Wisconsin Carbone Cancer Center Pancreas Pilot Grant program.References
1. Porembka, M.R., et al., Radiologic and intraoperative detection of need for mesenteric vein resection in patients with adenocarcinoma of the head of the pancreas. HPB (Oxford), 2011. 13(9): p. 633-42.
2. Kluger, M.D., et al., Resection of Locally Advanced Pancreatic Cancer without Regression of Arterial Encasement After Modern-Era Neoadjuvant Therapy. J Gastrointest Surg, 2018. 22(2): p. 235-241.
3. Ghaneh, P., et al., The Impact of Positive Resection Margins on Survival and Recurrence Following Resection and Adjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma. Ann Surg, 2019. 269(3): p. 520-529.
4. Strobel, O., et al., Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg, 2017. 265(3): p. 565-573.
5. Verbeke, C.S., Resection margins in pancreatic cancer. Pathologe, 2013. 34 Suppl 2: p. 241-7.
6. Verbeke, C.S. and I.P. Gladhaug, Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg, 2012. 99(8): p. 1036-49.
7. Verbeke, C.S. and K.V. Menon, Redefining resection margin status in pancreatic cancer. HPB (Oxford), 2009. 11(4): p. 282-9.
8. Kinner, S., et al., Contrast-Enhanced Abdominal MRI for Suspected Appendicitis: How We Do It. AJR Am J Roentgenol, 2016. 207(1): p. 49-57.
9. Rendell, V., Timothy J. Colgan, Gesine Knobloch, Matthias R. Muhler, Agnes G Loeffler, Rashmi M. Agni, Krisztian Kovacs, Emily R. Winslow, Scott B. Reeder, Direct Radiologic-Pathologic Correlation of Liver Lesions with an MR-Compativle Sectioning and Localization Device. 2019: ISMRM Annual Meeting and Exhibition: 2019.
Figures
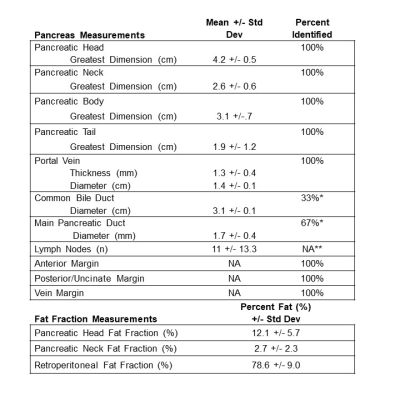
Table 1: Percent identification, anatomic measurements and fat fraction
*The common bile duct and main pancreatic duct were unable to be identified secondary to pathologic sectioning prior to imaging in 4/6 and 2/6 specimens, respectively. **Could not be verified because pathologic examination of all nodes was not conducted for each specimen.
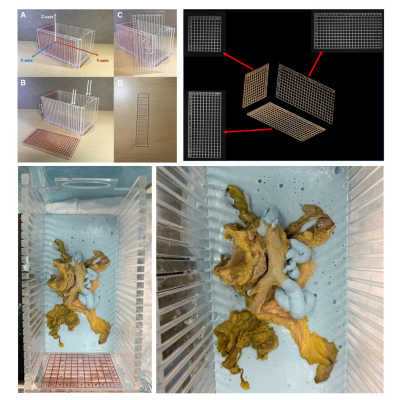
Figure 1: Radiologic Histologic Correlation Device
Clockwise from top left: Radiologic histologic correlation device (RHCD) with MRI visible grid depicting 3-dimensional axis; 3D grid axis as depicted in MR images allows for radiologic-histologic co-localization; cadaver pancreas specimen within alginate (blue) in the RHCD; a second view of a cadaver pancreas specimen within the RCHD.
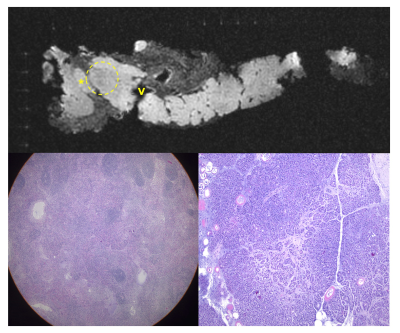
Figure 2: T1w, axial view of pancreas specimen with corresponding histology
Ex vivo MRI of detailed pancreatic head anatomy within the adjacent RHCD grid including peripancreatic lymph node (*), portal vein (“V”), region of hypo-attenuation (circle) within the pancreas. The bottom right is the histology section of the area of hypo-attenuation (circle) consistent with autolysis surrounded by normal acinar cells. The bottom left is the histology section from the lymph node (*) consistent with a reactive lymph node with prominent germinal centers.
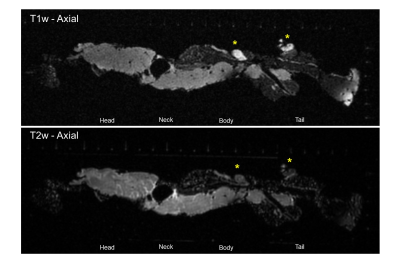
Figure 3: T1w vs T2w axial view of peripancreatic node and the portal vein
Ex vivo MRI of detailed pancreas anatomy. Image demonstrates lymph nodes (*) more easily seen in the T1w vs T2w image, the portal vein (“V”), pancreas head, neck, body, tail, and peripancreatic fat
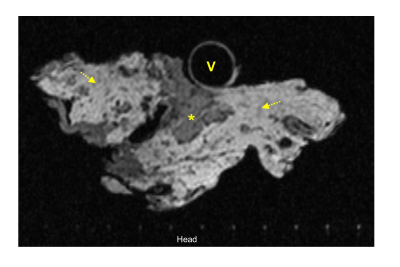
Figure 4: T2w axial view of portal vein, pancreas head, and peripancreatic fat
Ex vivo MRI of detailed pancreas head anatomy. Image demonstrates the portal vein (V), pancreas head directly abutting the portal vein (*), fatty infiltrated pancreas head (*), peripancreatic fat (arrows), with the 1cm RHCD grid marks on bottom of image.