0835
Amide Proton Transfer Imaging of Rectal Cancer: Baseline values and Feasibility for Predicting Tumor Pathological Features1Radiology, Union Hospital, Tongji Medical College,Huazhong University of Science and Technology, Wuhan, China
Synopsis
We speculate that APT value may be a useful biomarker for assessing rectal pathological characteristics, which could have a potential impact on the clinical therapeutic strategies for patients. We have established a baseline for the APT values in rectal cancer, which was shown significantly correlated with pathologic features (eg. tumor grade and Ki-67 index).
INTRODUCTION
The standard treatment for patients with locally advanced rectal cancer (LARC) is neoadjuvant CRT followed by total mesorectal excision [1], while tumor staging with rectal magnetic resonance (MR) imaging is premise. Since morphological details are insufficient and diffusion-weighted MR imaging appears to be controversial [2] for better staging of rectal cancer, a new imaging biomarker to grade rectal cancer more accurately is desirable. Amide proton transfer-weighted (APTw) imaging is a molecular MRI technique that generates image contrast based predominantly on the amide protons in mobile cellular proteins and peptides that are endogenous in tissue, which have already shown potential application in diseases of central nervous system [3]. Recently, several exploratory APT-related researches on rectal cancer are reported [4-5], while there has been no report on the reproducibility of APTw imaging in rectal cancer, nor research on the capability of APTw imaging in LARC detection. We thus conducted the present study to determine whether APTw imaging is repeatable in rectal cancer and whether APTw imaging can predict tumor pathological features for LARC.METHODs AND MATERIALs
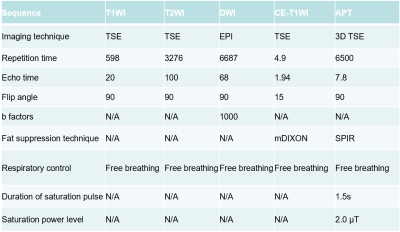
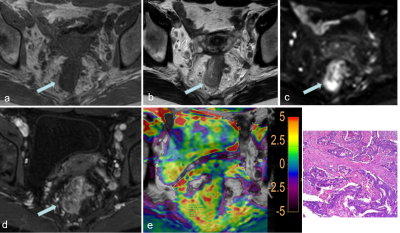
Twenty-three patients with suspected rectal mass were prospectively recruited into this institutional review board-approved study. All participants were scanned at 3.0T (Ingenia CX, Philips Healthcare, Best, the Netherlands) using standard rectal protocols (T1WI, T2WI, DWI, contrast-enhanced) and APTw-imaging (acquired with 3D turbo-spin-echo sequence), imaging parameters listed in Table 1. Total mesorectal excision was performed on 17 patients (6 patients with locally advanced rectal cancer who received neoadjuvant chemoradiation were excluded), 15 patients with surgical-pathological rectal cancer were finally included (another 2 rectal tumors proved as rectal adenoma were further excluded) and the relevant pathological features were evaluated. APT values [6] were measured by two radiologists independently drawing ROIs on a commercially available post-processing workstation (IntelliSpace Portal, Philips Healthcare, Best, the Netherlands). Polygonal ROIs were placed in the solid component of a tumor on the T2WI image, avoiding cystic, large necrotic, or hemorrhagic components, and copied onto the APTw image by each radiologist. The size of ROIs were draw ≥260 mm2 (≥80 pixels) and as large as possible, with exemplary ROIs shown in Figure 1. Pathological features including tumor grade and Ki-67 index were evaluated. Inter-observer agreement on APT values was analyzed using intraclass correlation coefficient (ICC). APT values were compared between the two groups classified by pathological grade using independent sample t-test, while the correlation between APT values and Ki-67 index was evaluated using Pearson correlation analysis.RESULTS
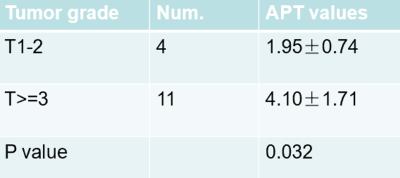
Representative images from one patient, including T2W, T1W, DWI, contrast-enhanced, and a fusion of APTw and T2W images, were demonstrated in Figure 1. APT values of all tumors was 3.53±1.78(%‚ range from1.03 to7.80). The ICC of APT values was 0.961(p<0.05) between the 2 radiologists. Four rectal tumors graded as T1 and T2 were classified in lower grade group, while 11 rectal tumors graded as T3 or more advanced were classified in LARC group, results shown in Table 2. APT values between the group of T1-2 and T≥3 were significantly different(p<0.05). The Ki-67 index was significantly associated with APT value (R=0.793,,p<0.05).DISCUSSION and CONCLUSION
Rectal cancer is usually very proliferative and possesses high considerable potential in long-distance metastases [7-8], which likely lead to an abundant pool of mobile cellular proteins and peptides. We have established a baseline for the APT values in rectal cancer, which was shown significantly correlated with pathologic tumor grade and Ki-67 index. We speculate that APT value may be a useful biomarker for assessing rectal pathological characteristics, which could have a potential impact on the clinical therapeutic strategies for patients, and this needs to be validated in a further study with larger sample size. Meanwhile, a baseline of APT value needs to be established for healthy rectum, which remains challenging due to the susceptibility effects and the limited thickness in normal tissue.Acknowledgements
We thank Prof. Jiazheng Wang, Philips health, for providing technical support.References
1. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Rectal Cancer version 2.2017.https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed December 15, 2017.
2. Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumor response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur. Radiol. 2011; 21: 987–95. 3. Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 2003; doi: 10.1038/nm907.
4. Nishie A, Takayama Y, Asayama Y, Ishigami K, Ushijima Y, Okamoto D, Fujita N, Tsurumaru D, Togao O, Sagiyama K, Manabe T, Oki E, Kubo Y, Hida T, Hirahashi-Fujiwara M, Keupp J, Honda H. Amide proton transfer imaging can predict tumor grade in rectal cancer2018;51:96-103.
5. Nishie A, Asayama Y, Ishigami K, Ushijima Y, Takayama Y, Okamoto D, Fujita N, Tsurumaru D, Togao O, Sagiyama K, Manabe T, Oki E, Kubo Y, Hida T, Hirahashi-Fujiwara M, Keupp J, Honda H. Amide proton transfer imaging to predict tumor response to neoadjuvant chemotherapy in locally advanced rectal cancer. Journal of Gastroenterology and Hepatology 2019;34 :140–146.
6. He YL, Li Y, Lin CY, et al. Three-dimensional turbo-spin-echo amide proton transfer-weighted MRI for cervical cancer: a preliminary study. J Magn Reson Imaging 2019; doi: 10.1002/jmri.26710
7. Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
8. Yang, Y.; Huang, X.; Sun, J.; Gao, P.; Song, Y.; Chen, X.; Zhao, J.; Wang, Z. Prognostic value of perineural invasion in colorectal cancer: A meta-analysis. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2015, 19, 1113–1122.
Figures