0830
Delta Radiomic Features from serial bi-parametric MRI are associated with biopsy upgrading of prostate cancer patients on Active Surveillance1Case Western Reserve University, Cleveland, OH, United States, 2UH Cleveland Medical Center, Cleveland, OH, United States, 3Case Western Reserve University School of Medicine, Cleveland, OH, United States
Synopsis
Serial MRI allows for non-invasive monitoring of prostate cancer patients on Active Surveillance (AS). However, repeat biopsies continue to be defacto standard for AS monitoring due to limitations of MRI. In this study, we sought to compute delta changes in radiomic features on serial bi-parametric MRI and evaluate their associations with biopsy upgrading. We observed that delta radiomic features that quantify underlying spatial, gradient based heterogeneity were associated with biopsy upgrading. On univariable and multivariable analysis with routine clinical variables, we observed that none of the clinical variables were significant while delta radiomic classifier predictions were significant and independently predictive.
Introduction
Active Surveillance (AS) is increasingly emerging1 as a viable option for prostate cancer (PCa) patients who are at low risk of disease progression and do not need immediate treatment. AS involves periodic monitoring of patients via repeat prostate specific antigen (PSA) measurements and biopsies, which however are expensive and cause immense discomfort2. Serial MRI3 may allow for non-invasive monitoring of AS patients and has been shown to add incremental benefit. Bi-parametric MRI (bpMRI) including T2-weighted (T2W) and diffusion weighted sequences (DWI) is gaining importance4 as a rapid imaging protocol for PCa assessment. Radiomics from prostate bpMRI quantify the underlying heterogeneity and have been shown to be associated with PCa aggressiveness5–7. In this study, we hypothesize that differences in radiomics or delta radiomics (DeRads) across serial MRI may capture signatures associated with progression of PCa. The goal of this study is to evaluate associations between DeRads and upgrading on systematic biopsy following initial screening of PCa patients on AS.Methods
This retrospective, IRB approved and HIPAA compliant study comprised N = 32 PCa patients on AS who underwent a screening 3T multi-parametric MRI (mpMRI) scan followed by a second MRI scan (mean interval = 16 months). They underwent transrectal, ultrasound guided systematic biopsy after screening MRI and subsequently when presented with elevated PSA levels. Patients were considered to have a biopsy upgrade (AS+) when their subsequent biopsy resulted in higher Gleason Grade Group (GGG) and no upgrade (AS–) when their GGG remained the same or lower. Biopsy used to determine upgrading was after or close to the second MRI scan. An experienced radiologist delineated PCa regions of interest (ROIs) on bpMRI from baseline and second MRI scan. Radiomic features including 1st and 2nd order statistics, Haralick, Gabor, Co-occurrence of local anisotropic gradient orientations (CoLlAGe8) and Laws were extracted from ROIs and distribution statistics (mean, variance, skewness and kurtosis) of radiomics within ROIs were computed. DeRads were computed as difference between radiomic features within ROIs on serial bpMRI scans (screening and second MRI). Support vector machine (SVM) classifier (CΔ) was trained with DeRads in a 3-fold cross validation framework. Association between DeRads and biopsy upgrading was assessed for statistical significance (p<0.05) using Wilcoxon rank-sum test and classification performance in terms of area under the receiver operating characteristics (AUC) curve. Comparison with routine clinical variables including age, PSA, PIRADS-v2 (Prostate Imaging Reporting and Data System), GGG and race was performed in terms of univariable and multivariable analysis.Results
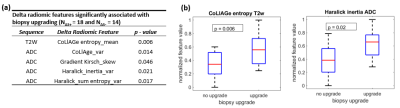
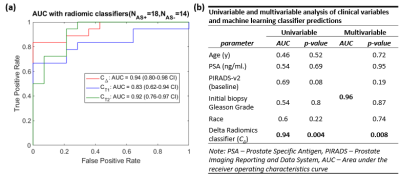
Of the N = 32 patients, 18 experienced biopsy upgrading and 14 had no upgrade. Radiomic features including CoLlAGe from T2W and gradient, Haralick from ADC showed statistically significant differences between AS+ and AS– patients (Figure 1). Machine learning classifiers trained using radiomic features from baseline (CT1) and subsequent MRI (CT2), resulted in cross-validation AUCs of 0.83, 95% Confidence Interval (CI): (0.62-0.94 CI) and 0.92, 95% CI: (0.76-0.97) respectively in distinguishing AS+ and AS– patients. Classifier CΔ resulted in cross-validation AUC = 0.94, 95%CI: (0.80-0.98) which was significantly higher (p<0.05) than CT1 and CT2 (Figure 2). On univariable analysis, none of the clinical variables were able to significantly differentiate AS+ and AS– patients. On multivariable analysis, CΔ was a significant predictor of biopsy upgrading and provided independent value compared to other clinical variables (Figure 2).Discussion
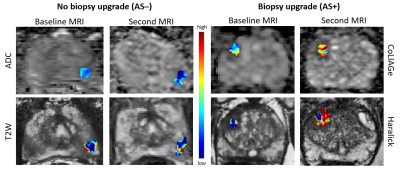
Radiomic features quantify underlying heterogeneity on imaging that is not discernible on routine imaging. Changes in radiomic feature of prostate cancer lesions or delta radiomics (DeRads) may help quantify changes in tissue morphology that could potentially capture information related to disease progression in prostate cancer (PCa) patients on Active Surveillance (AS). We observed DeRads to show significant differences between patients with (AS+) and without (AS–) biopsy upgrading. CoLlAGe features quantify intensity gradient based heterogeneity while gradient and Haralick features quantify spatial intensity distribution based heterogeneity. As shown in Figure 3, there was increased heterogeneity in AS+ patients on second MRI compared to AS- patients. A majority of significant DeRads features were derived from ADC maps which was also observed in previous studies9,10 that showed texture analysis of ADC maps to be beneficial in predicting pathological upgrading of PCa lesions. The classification performance using DeRads was significantly better than using radiomics from baseline or second MRI scan individually. This suggests that change in radiomic features may be better associated with biopsy upgrading than individual MRI scans. On univariable analysis, PIRADS-v2 from baseline MRI was the only clinical variable with high AUC and was nearly significant (p=0.08). A recent study11 has also shown that higher PIRADS is associated with increased risk of biopsy upgrading. We observed that delta radiomics classifier predictions were significant and also independent predictor of upgrading on multivariable analysis further suggesting that DeRads may encode signatures associated with disease progression that may not be captured by routine clinical variables. Our study is limited by a smaller sample size, lack of an independent validation set and variations in follow up of AS patients. These will be addressed in our future work which is part of our ongoing study.Conclusion
Delta radiomics features derived from serial bi-parametric MRI of the prostate may be predictive of biopsy upgrading in Active Surveillance patients.Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers 1U24CA199374-01, R01CA202752-01A1R01CA208236-01A1R01 CA216579-01A1R01 CA220581-01A11U01 CA239055-01
National Institute for Biomedical Imaging and Bioengineering 1R43EB028736-01
National Center for Research Resources under award number 1 C06 RR12463-01
VA Merit Review Award IBX004121A from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service
The DoD Breast Cancer Research Program Breakthrough Level 1 Award W81XWH-19-1-0668
The DOD Prostate Cancer Idea Development Award (W81XWH-15-1-0558)
The DOD Lung Cancer Investigator-Initiated Translational Research Award (W81XWH-18-1-0440)The DOD Peer Reviewed Cancer Research Program (W81XWH-16-1-0329)
The Ohio Third Frontier Technology Validation FundThe Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering and The Clinical and Translational Science Award Program (CTSA) at Case Western Reserve University.
The DoD Prostate Cancer Research Program Idea Development Award W81XWH-18-1-0524
References
- Chen RC, Rumble RB, Loblaw DA, Finelli A, Ehdaie B, Cooperberg MR, Morgan SC, Tyldesley S, Haluschak JJ, Tan W, Justman S, Jain S. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol. 2016 Jun 20;34(18):2182–2190. doi:10.1200/JCO.2015.65.7759
- Brooks JV, Ellis SD, Morrow E, Kimminau KS, Thrasher JB. Patient Factors That Influence How Physicians Discuss Active Surveillance With Low-Risk Prostate Cancer Patients: A Qualitative Study. Am J Mens Health. 2018;12(5):1719–1727. doi:10.1177/1557988318785741 PMID: 29973123 PMCID: PMC6142114
- Felker ER, Wu J, Natarajan S, Margolis DJ, Raman SS, Huang J, Dorey F, Marks LS. Serial Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: Incremental Value. J Urol. 2016 May;195(5):1421–1427. doi:10.1016/j.juro.2015.11.055
- Steinkohl F, Pichler R, Junker D. Short review of biparametric prostate MRI. Memo. 2018;11(4):309–312. doi:10.1007/s12254-018-0458-1 PMID: 30595756 PMCID: PMC6280777
- Shiradkar R, Ghose S, Jambor I, Taimen P, Ettala O, Purysko AS, Madabhushi A. Radiomic features from pretreatment biparametric MRI predict prostate cancer biochemical recurrence: Preliminary findings. J Magn Reson Imaging JMRI. 2018 May 7; doi:10.1002/jmri.26178 PMID: 29734484
- Ginsburg S, Ali S, Lee G, Basavanhally A, Madabhushi A. Variable importance in nonlinear kernels (VINK): classification of digitized histopathology. Med Image Comput Comput-Assist Interv MICCAI Int Conf Med Image Comput Comput-Assist Interv. 2013. p. 238–245. PMID: 24579146
- Viswanath SE, Bloch NB, Chappelow JC, Toth R, Rofsky NM, Genega EM, Lenkinski RE, Madabhushi A. Central gland and peripheral zone prostate tumors have significantly different quantitative imaging signatures on 3 Tesla endorectal, in vivo T2-weighted MR imagery. J Magn Reson Imaging JMRI. 2012 Jul;36(1):213–224. doi:10.1002/jmri.23618 PMID: 22337003 PMCID: PMC3366058
- Prasanna P, Tiwari P, Madabhushi A. Co-occurrence of Local Anisotropic Gradient Orientations (CoLlAGe): A new radiomics descriptor. Sci Rep. 2016 Nov 22;6:37241. doi:10.1038/srep37241 PMID: 27872484 PMCID: PMC5118705
- Rozenberg R, Thornhill RE, Flood TA, Hakim SW, Lim C, Schieda N. Whole-Tumor Quantitative Apparent Diffusion Coefficient Histogram and Texture Analysis to Predict Gleason Score Upgrading in Intermediate-Risk 3 + 4 = 7 Prostate Cancer. AJR Am J Roentgenol. 2016 Apr;206(4):775–782. doi:10.2214/AJR.15.15462 PMID: 27003049
- Sadoughi N, Krishna S, McInnes MDF, Flood TA, Breau RH, Morash C, Schieda N. ADC Metrics From Multiparametric MRI: Histologic Downgrading of Gleason Score 9 or 10 Prostate Cancers Diagnosed at Nontargeted Transrectal Ultrasound-Guided Biopsy. AJR Am J Roentgenol. 2018;211(3):W158–W165. doi:10.2214/AJR.17.18958 PMID: 29995495
- Kornberg Z, Cowan JE, Westphalen AC, Cooperberg MR, Chan JM, Zhao S, Shinohara K, Carroll PR. Genomic Prostate Score, PI-RADSTM version 2 and Progression in Men with Prostate Cancer on Active Surveillance. J Urol. 2019;201(2):300–307. doi:10.1016/j.juro.2018.08.047 PMID: 30179620
Figures