0826
Cortical Surface Spectral Matching of the Fetal Brain Pre and Post Fetal Surgery for Open Spina Bifida1Institute for Women's Health and Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom, 2School of Biomedical Engineering and Imaging Sciences (BMEIS), King's College London, London, United Kingdom, 3Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom, 4Department of Radiology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium, 5Department of Obstetrics and Gynaecology, University Hospitals Katholieke Universiteit (KU) Leuven, Leuven, Belgium, 6University Hospitals KU Leuven, Leuven, Belgium
Synopsis
Comprehensive evaluation of the fetal central nervous system (CNS) is required to select the most suitable candidates, to counsel parents about fetal spina bifida surgery, and to monitor post-op response. In children and adolescents with spina bifida, MRI assessment of gyrification correlates with motor and cognitive function. Our aim is to determine if MRI can quantify fetal brain gyrification and folding before and after fetal spina bifida surgery. If successful, mapping the gyrification changes to different lobes in the brain may prove useful in the prediction of motor and cognitive function after fetal surgery.
Introduction
Fetal surgery has become a clinical reality, even for non-lethal conditions such as open spina bifida (OSB). Myelomeningocele (MMC), is the most common form of OSB where the spinal cord extrudes into a cereberospinal fluid (CSF) filled sac1,2. It is associated with brain anomalies such as hindbrain herniation and variable degrees of ventriculomegaly. Prenatal repair yields better outcomes compared to postnatal surgery3. Nonetheless, mechanical tissue damage of brain parenchyma due to ventriculomegaly and damage to the neural tracts, lead to abnormal white matter development, as demonstrated by diffusion weighted imaging studies4-9.This may lead to altered gyrification patterns in MMC patients. Gyrification, measured by magnetic resonance imaging (MRI), correlates with motor and cognitive function in infants, children and adolescents with MMC who have undergone postnatal closure6. Evaluation of cognitive and motor function in MMC fetuses who have had prenatal surgery, is performed only after birth, with deficits becoming more evident with increasing age. Clinicians therefore urgently need early fetal brain imaging methods that can predict the cognitive and motor challenges that MMC fetuses may encounter after birth. We aim to demonstrate that longitudinal quantitative MRI measurement of cortical gyrification is possible, before and after MMC repair. We will also demonstrate the curvature (curvedness and shape index) of MMC cerebellum and ventricles before and after surgery.Methods
T2-weighted single-shot fast spin-echo (SSFSE) was performed of the fetal brain in multiple orientations containing an axial, coronal and sagittal plane,with 3mm slice thickness, on women with a fetus with isolated MMC, both before surgery (n=12, 23+6 +/-1+7 weeks, (22+1–25+6)) and after surgery (n=12, 26+1 +/- 1+3 weeks, (24+1– 29+4)). Total acquisition time was thirty-forty minutes. Fetuses affected by aneuploidy or with structural anomalies outside the central nervous system (CNS) were excluded. A novel automated super resolution reconstruction (SRR) algorithm10,11was used to build 3D volumes of the fetal brain. Brain masks for pre-operative SRR volumes were resampled from their corresponding post-operative MMC masks after affine and non-rigid alignment. We automatically segmented white matter, ventricles, and cerebellum using template brain segmentations12,13. All masks where manually corrected and meshes were generated using ITK-Snap14. We make use of a surface-based spectral matching technique12 to find a longitudinal correspondence of these cerebral structures before and after fetal surgery. A rigid coherent point drift algorithm was applied to find an initial correspondence for the intrasubject cortical, cerebellar and ventricle regions before and after surgery. Joint spectral matching (JSM) was then used to find the correspondence for the intrasubject cerebral structures at those two different time points. In JSM a dual layered graph was produced whereby layers correspond to the surface of the white matter, cerebellum or ventricles of each subject. The correspondence links from the initial intrasubject matching, connecting both layers to produce a set of shared eigenmodes of the surfaces. After mapping the post-operative surface to the pre-operative surface using JSM, we computed the change in parameters at the vertex of each mesh to explore longitudinal cortical gyrification, and curvature (curvedness and shape index) of the cerebral structures12.Results
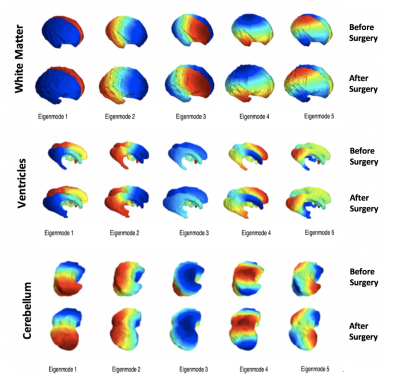
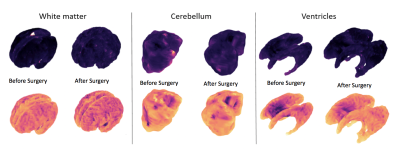
Figure 1 illustrates five spectral modes for the white matter, ventricles and cerebellum of a fetus before surgery (24 weeks), and therafter (26 weeks). Although the meshes are quite different in the three-dimensional space, with respect to different levels of folding, variation in shape, surface area and volume, they have similar representations in the spectral domain.Figure 2 shows the curvedness and shape index in the white matter, cerebellum and ventricles before and after fetal MMC surgery. JSM allows us to map the mean curvatures of each mesh to compute changes in the mean and to generate the shape index.
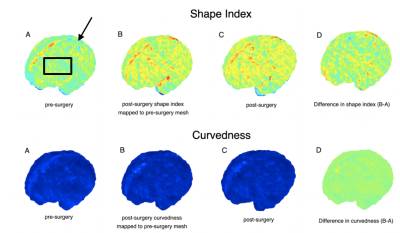
Figure 3 shows maps of white matter mean curvature of a fetus pre and post-surgery. Positive values are depicted in red/yellow and represent gyri (convex structures), and negative values in blue represent sulci (concave) structures. JSM allows mapping of mean curvatures from the post-op to the pre-op space, computing the changes in mean curvature between these two time points in the pre-op space.
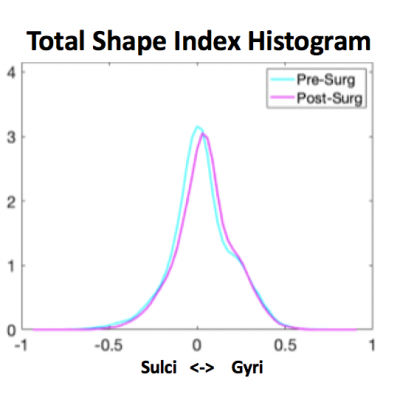
Figure 4 illustrates the total shape index histogram for white matter, showing the differences in gyri and sulci between the pre and post-operative MMC brain. It demonstrates the appearance of more gyri after surgery taking into account increasing gestational age.
Discussion and conclusion
Surface-based matching provides additional information about changes in growth and gyrification of the fetal brain compared to measurement of total volume and shape change. This may be useful in evaluating changes in cerebral growth of MMC fetuses before and after fetal surgery. Spectral graph matching is a promising tool for matching shapes with significant differences in cortical folding, surface area, and volume, but with similar representations in the spectral domain such as depicted with MMC fetuses before and after surgery12,15. Future work may be able to better explore the physiological and mechanical properties contributing to the differences observed in brain growth and development in the context of fetal surgery.Acknowledgements
This work is part of the Guided Instrumentation of Fetal Therapy and Surgery (GIFT-Surg) project, funded by the Wellcome Trust [203148/Z/16/Z; 203145Z/16/Z; WT101957] and Engineering and Physical Sciences Research Council (EPSRC) [NS/A000049/1; NS/A000050/1; NS/A000027/1; EP/L016478/1] Wellcome/EPSRC centre for Interventional and Surgical Sciences (WEISS)References
1. Rethmann, C., et al., Evolution of posterior fossa and brain morphology after in utero repair of open neural tube defects assessed by MRI. Eur Radiol, 2017. 27(11): p. 4571-4580.
2. Zarutskie, A., et al., Prenatal brain imaging for predicting need for postnatal hydrocephalus treatment in fetuses that had neural tube defect repair in utero. Ultrasound Obstet Gynecol, 2019. 53(3): p. 324-334.
3. Adzick, N.S.T., E.A.; Spong, C. Y.; Brock III, J. W.; Burrows, P. K.; Johnson, M. P.; Howell, R. N.; Farrell, J. N.; Dabrowiak, M.E.; Sutton, L.N.; Gupta, N.; Tulipan, N.B.; D’Alton, M.E.; Farmer, D.L., A Randomised Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N Engl J Med, 2011. 364: p. 993-1004.
4. Juranek, J., et al., Neocortical reorganization in spina bifida. Neuroimage, 2008. 40(4): p. 1516-22.
5. Juranek, J. and M.S. Salman, Anomalous development of brain structure and function in spina bifida myelomeningocele. Developmental Disabilities Research Reviews, 2010. 16(1): p. 23-30.
6. Treble, A., et al., Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb Cortex, 2013. 23(10): p. 2357-69.
7. Hasan, K.M., et al., White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: a diffusion tensor tractography study of the association pathways. J Magn Reson Imaging, 2008. 27(4): p. 700-9.
8. Mignone Philpott, C., et al., Diffusion-weighted imaging of the cerebellum in the fetus with Chiari II malformation. AJNR Am J Neuroradiol, 2013. 34(8): p. 1656-60.
9. Woitek, R., et al., Fetal diffusion tensor quantification of brainstem pathology in Chiari II malformation. Eur Radiol, 2016. 26(5): p. 1274-83.
10. Ebner, M., et al., An Automated Localization, Segmentation and Reconstruction Framework for Fetal Brain MRI, in Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. 2018. p. 313-320.
11. Ebner, M., et al., An automated framework for localization, segmentation and super-resolution reconstruction of fetal brain MRI. NeuroImage, 2019.
12. Orasanu, E., et al., Cortical folding of the preterm brain: a longitudinal analysis of extremely preterm born neonates using spectral matching. Brain Behav, 2016. 6(8): p. e00488.
13. Kuklisova-Murgasova, M., et al., A dynamic 4D probabilistic atlas of the developing brain. Neuroimage, 2011. 54(4): p. 2750-63.
14. Yushkevich, P.A., et al., User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage, 2006. 31(3): p. 1116-28.
15. Lomabert, H., Spopring J., and Siddiqi K., Diffeomorphic spectral matching of cortical surafaces. Inf Process Med Imaging, 2013. 7917: p. 376-289.
Figures