0825
Clinical Diffusion Time Dependency of Breast Tumors and Associations with Prognostic Factors1Diagnostic Imaging and Nuclear Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan, 2Clinical Innovative Medicine, Institute for Advancement of Clinical and Translational Science, Kyoto University Hospital, Kyoto, Japan, 3Siemens Healthcare K.K., Tokyo, Japan, 4Siemens Healthcare GMBH, Erlangen, Germany, 5Breast Surgery, Kyoto University Graduate School of Medicine, Kyoto, Japan
Synopsis
We investigated the variation of ADC values obtained at diffusion times that are clinically available for differentiation of human breast tumors. The ADC values in both malignant and benign breast tumors decreased with increased diffusion time, and a larger change in ADC values was found in malignant tumors. The significant association found between ADC change and Ki-67 expression might indicate the potential of diffusion time-dependent ADC values as a tool to differentiate these prognostic biomarkers and assess tumor heterogeneity without the need for contrast agents.
Introduction
Diffusion MRI is widely used for detection and characterization of breast lesions without the need for contrast agents, and many previous studies have demonstrated the clinical utility of ADC in differentiation between malignant and benign breast lesions or breast tumor subtypes linked to prognostic factors.1 Furthermore, studies have shown changes in ADC values with different diffusion time and their utility in tumor characterization in the prostate, head and neck, and breast tumors.2-5 Increased diffusion hindrance is expected at longer diffusion time as more water molecules collide with microscopic obstacles within tissues, such as cell membranes, the density of which increases in cancer tissues. Improvements in MRI gradient hardware have allowed the acquisition of shorter diffusion times (such as oscillating gradient spin-echo (OGSE)) with some clinical scanners. Measuring ADC values at different diffusion times can provide important information about the degree of diffusion hindrance and in turn the nature of the lesions. It may also provide information about the different expression levels of prognostic biomarkers in human breast tumors. Thus, our purpose was to investigate the variability of clinical ADC measurements and their association with diffusion times using OGSE and pulsed gradient spin-echo (PGSE) in human breast tumors.Methods
OGSE and PGSE sequences were performed on a dedicated-breast polyvinylpyrrolidone phantom (covering typical ADC values from malignant to benign lesions6) and patients using a clinical 3-T system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with a dedicated 16-channel breast array coil. This prospective study investigated 72 breast tumors (36 malignant and 36 benign). Data acquisition utilized diffusion-weighted echo planar prototype sequences with b-values of 0 and 700 s/mm2. OGSE was performed with trapezoid cosine waveforms (frequency = 40 Hz; effective diffusion time (Deff) =5.1ms) and PGSE (Deff=96.6 ms); repetition time/echo time, 7,500ms/125ms; field of view, 330×330 mm2; matrix, 112×112; slice thickness, 3.0 mm; acquisition time averaged over 8 averages, 2.5 min for each, OGSE and PGSE. Two independent radiologists placed ROIs in lesions on the ADC maps, and mean ADC values were calculated. An ADC change was calculated as (ADCOGSE − ADCPGSE) / ADCOGSE×100 (%). Agreement of ADC values or ADC change between two independent radiologists was calculated using intraclass correlation coefficients (ICC). Wilcoxon test was used for the comparison of ADC values between different diffusion times. ADC values and ADC change with diffusion time were compared between samples that were positive and negative for expression of prognostic biomarkers (ER, PgR, Her2, Ki-67) using Mann-Whitney tests.Results
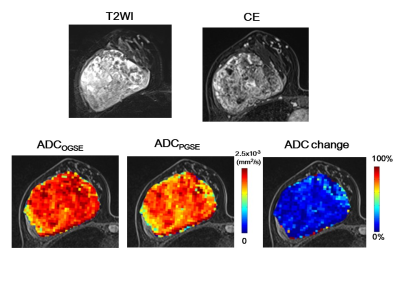
As expected, ADC values in PVP phantom showed no difference for Gaussian diffusion, validating the OGSE and PGSE protocols. Excellent agreement between independent readers was observed for ADC values and ADC change (ICC: 0.91–0.98), so each value was averaged for further analysis. The ADC values in malignant lesions were significantly lower than those in benign lesions at both diffusion times, and ADC values significantly decreased with increased diffusion times (P < 0.01 and < 0.01, Fig.1a). A significant association was found between ADC change and Ki-67 status (P < 0.05, Fig. 1b). No significant difference was found in other prognostic biomarkers or subtypes of breast cancer. Figures 2–3 demonstrate representative ADC maps at different diffusion times. A mild ADC decrease was observed at the center of the cancer, whereas ADC sharply decreased in the peripheral region (Fig. 2). In contrast, very small ADC changes were observed in a benign phyllodes tumor (Fig. 3) .The diagnostic performance of ADCPGSE was slightly better than that of ADCOGSE or ADC change, with no significant difference (AUC: 0.97, 0.94, and 0.96, respectively, Fig.4).
Discussion
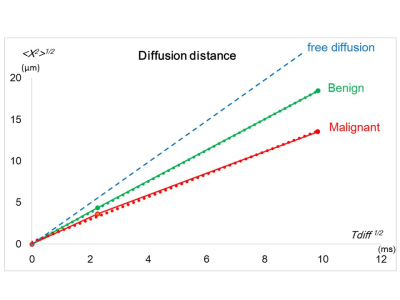
The observed decrease of ADC values with diffusion time in breast tumors was in agreement with the literature, as well as with our previous investigation.2-5 This confirms the hypothesis that diffusion hindrance increases with diffusion time in tumors, as more molecules hit many boundaries such as cell membranes that are water-permeable, to which ADC is highly sensitive.7 The lower ADC values observed in malignant than benign tumors suggest a dependence of water molecule displacement on tissue type, as shown in Fig. 5. The difference of apparent diffusion distance between malignant and benign tumors was smaller in OGSE than in PGSE, and using OGSE might diminish the contrast for differentiation of tumor type. The association of ADC change with Ki-67 expression might reflect differences in the proliferative activity of some tumors and help to assess prognostic biomarkers for breast cancer without the use of contrast agents.Conclusion
The diffusion time dependence of ADC measurements for differentiation of prognostic biomarkers in human breast tumors was investigated. The ADC values in breast tumors varied with diffusion time, suggesting the necessity of reporting diffusion times in corresponding studies. The significant association of ADC change with Ki-67 expression might help to differentiate these prognostic biomarkers, determine treatment plans, and assess tumor heterogeneity without the need for contrast agents.Acknowledgements
This work was supported by MEXT KAKENHI Grant No. 18K15588.References
(1) Iima M, Honda M, Sigmund EE, et al. Diffusion MRI of the breast: Current status and future directions. J Magn Reson Imaging. 2019 Sep 14.
(2)
Reynaud O et al.
Surface-to-volume ratio mapping of tumor microstructure using
oscillating gradient diffusion weighted imaging. Magn Reson
(3)
Lemberskiy G, Rosenkrantz AB, Veraart J et al.
Time-Dependent Diffusion in Prostate Cancer. Invest Radiol. 2017
Jul
(4) Iima M et al. Time makes the difference: Comparison of ADC values obtained with OGSE and PGSE sequences for differentiation of human breast tumors. ISMRM 2018.
(5)
Iima M et al. Time-dependent diffusion MRI to distinguish
malignant from benign head and neck tumors J Magn Reson Imaging. 2019
Jul
(6) Wagner F et al. Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms.PLoS One. 2017 Jun 19;12(6):e0179276.
(7)
Springer CS Jr1. Using 1H2O MR to measure and map
sodium pump activity in
Figures
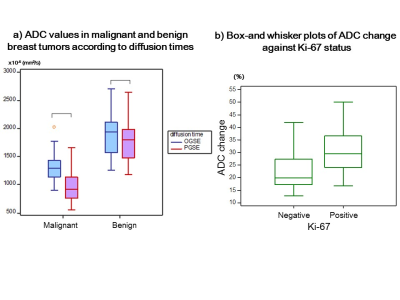
Figure 1a. ADC values in malignant and benign breast tumors according to diffusion times.
Figure 1b. Box-and-whisker plots of ADC change against Ki-67 status.