0817
The Application of High-resolution Multi-shot DWI with MUSE reconstruction in the Diagnosis of Active Inflammation in Crohn's Disease1The Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, China
Synopsis
Diffusion-weighted imaging(DWI) has been shown to be useful in evaluation of active bowel inflammation. Single-shot diffusion-weighted echo-planar imaging (ssDW-EPI) was commonly used in previous studies. However, ssDW-EPI suffers from geometric distortion and low spatial resolution. Moreover, the data acquisition of ssDW-EPI in bowel region become more difficult due to serve off-resonance effect. A recently developed multiplexed sensitivity-encoding (MUSE) framework can produce multi-shot DW-EPI (msDW-EPI) data with improved spatial resolution and reduced distortions. In this study, we demonstrated that the higher resolution and better overall image quality of msDW-EPI using MUSE framework potentially increased the accuracy in diagnosing active bowel inflammation.
Introduction
Magnetic resonance enterography (MRE) is a imaging modality to the diagnostic evaluation of active bowel inflammation in Crohn’s disease (CD), and its sensitivity and specificity were validated by various studies.1-11 In recent years, there has been evolving research interest in the role of diffusion weighted imaging (DWI) in the evaluation of active bowel inflammation. Single-shot diffusion-weighted echo-planar imaging (ssDW-EPI) was commonly used in these studies.2,12,13 ssDW-EPI can potentially replace/reduce the need for contrast-enhanced (CE) sequences in the assessment of active inflammation in CD, despite its limited spatial resolution compared to CE MRI.2,12 In addition, ssDW-EPI is susceptible to field inhomogeneity, leading to geometric distortion.14-16 The data acquisition of ssDW-EPI in bowel region become more difficult due to serve off-resonance effect. A recently developed multiplexed sensitivity-encoding (MUSE) framework can produce multi-shot DW-EPI (msDW-EPI) data with improved spatial resolution and reduced distortions.17,18 In this study, we proposed to improve the resolution and image quality of bowel DWI using MUSE framework, and evaluate its performance for diagnosing active inflammation in CD.Material and methods
Populations: Eighty-six patients with clinically suspected Crohn’s disease who had undergone MRE for evaluation of disease status were included. Each patient underwent contrast enhanced 3D T1-weighted images(T1WI), coronal and transverse T2WI, coronal and transverse T1WI, conventional ssDW-EPI (matrix of 128x128), and msDW-EPI (matrix of 192x192 with 4-shot acquisition). Sixty-three patients were diagnosed with active bowel inflammation from CE sequences, which was regarded as the standard reference. The other 23 patients did not have radiological evidence of active inflammation on CE sequences and served as the control group.Reconstruction of msDW-EPI Data: The raw data of msDW-EPI acquisition were transferred to a workstation for image reconstruction. The reconstruction pipeline is identical to original MUSE framework, including Nyquist ghost correction and measurement of inter-shot phase variation. The MUSE reconstructed images were exported to DICOM format for further analysis.
Image Analysis: For both patient groups, diagnostic evaluation of active bowel inflammation and evaluation of image quality were performed for ssDW-EPI and msDW-EPI data. For assessment of the image quality, 1) image resolution, 2) degree of geometric distortion, 3) impact of artifacts on image quality and diagnostic evaluation, and 4) overall image quality were assessed with a 5-point Likert scale.19 Images were independently reviewed by two board-certified radiologists, who were blinded to the type of DWI.
Statistical Analysis: Inter-rater reliability of image quality analysis was tested using the intraclass correlation coefficient (ICC).19 An ICC less than 0.50 indicated poor, between 0.50 to 0.75 moderate, between 0.75 and 0.90 good, and greater than 0.90 excellent.20 Two-tailed t-tests were performed to detect differences in image quality between ssDW-EPI and msDW-EPI. Statistical analyses were performed using SPSS.
Results
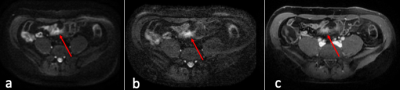
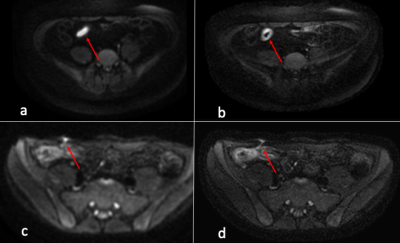
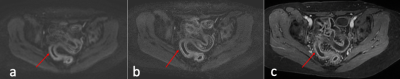
Diagnostic Evaluation: ssDW-EPI and msDW-EPI respectively identified 57 and 62 patients out of the 63 patients diagnosed with active bowel inflammation. False negative in ssDW-EPI and msDW-EPI were 6 and 1, respectively. There were no false positive in the control group for both imaging techniques. Results are shown in Table 1. Figure 1 illustrates a case diagnosed negative from ssDW-EPI, but positive on msDW-EPI.Image quality: Results of image quality ratings are summarized in Table 2. Image resolution and overall image quality were significantly better on msDW-EPI compared to ssDW-EPI (P<0.0001), with prefect agreement between two raters. Fig 2 and 3 show example images. Scores for image distortion and artifacts also favored msDW-EPI over ssDW-EPI. Inter-rater reliability for image quality was good for image resolution (ICC 0.881; 95% Confidence Interval (CI) 0.836-0.914) and overall image quality(ICC 0.822; 95% CI 0.753-0.871), and excellent for geometry distortion (ICC 0.957; 95% CI 0.941-0.969), artifacts influence on image quality (ICC 0.921; 95% CI 0.889-0.943) and artifacts influence on diagnostic evaluation (ICC 0.930; 95% CI 0.904-0.948). These are summarized in Table 2.
Discussion
In this study, we have demonstrated that the higher resolution and better overall image quality of msDW-EPI could potentially increase the accuracy in diagnostic evaluation of active bowel inflammation in CD. This includes the detection of bowel wall thickening, hyperenhancement, fistulation and other conditions (Figs. 2 and 3). Prior literatures have reported similar sensitivity and specificity of ssDW-EPI and CE-MRI.12,13 Our results showed that msDW-EPI could further improve the sensitivity and accuracy of DWI in the diagnostic evaluation of patients with active bowel inflammation in CD.There are two limitations for this study. First, the findings described on individual MRI reports were used as standard reference, rather than cross-correlation with the results of other diagnostic tests such as endoscopy. Second, CE-MRI findings were not internally validated in the study population due to the lack of histological results. These were mainly due to the fact that the diseased bowel segments were located in small intestine and beyond the reach of endoscopy for larger portion of patients, and that surgery is no longer commonly performed.
Conclusion
Multi-shot DW-EPI produces superior image quality and resolution compared to single-shot DW-EPI, and demonstrates promise in improving the accuracy of diagnostic evaluation of active bowel inflammation in Crohn’s disease.Acknowledgements
The project was partially supported by grants from Hong Kong Research Grant Council (GRF HKU17138616 and GRF HKU17121517) and Hong Kong Innovation and Technology Commission (ITS/403/18), and the donations from Jenssen Pharmaceuticals.References
1. Sinha R, Murphy P, Hawker P, et al. Role of MRI in Crohn's Disease. Clin Radiol. 2009;64:341-352.
2. Neubauer H, Pabst T, Dick A, et al. Small-Bowel MRI in Children and Young Adults with Crohn Disease: Retrospective Head-to-Head Comparison of Contrast-Enhanced and Diffusion-Weighted MRI. Pediatr Radiol. 2013;43:103-114.
3. Darbari A, Sena L, Argani P, et al. Gadolinium-enhanced magnetic resonance imaging: a useful radiological tool in di- agnosing pediatric IBD. Inflamm Bowel Dis. 2004;10:67-72.
4. Laghi A, Borrelli O, Paolantonio P, et al. Contrast enhanced magnetic resonance imaging of the terminal ileum in chil- dren with Crohn’s disease. Gut. 2003;52:393-397.
5. Masselli G, Casciani E, Polettini E, et al. Assessment of Crohn’s disease in the small bowel: prospective comparison of magnetic resonance enteroclysis with conventional enteroclysis. Eur Radiol. 2006;16:2817-2827.
6. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64-79.
7. Horsthuis K, Stokkers P, Stoker J. Detection of inflammatory bowel disease: diagnostic performance of cross-sectional imaging modalities. Abdom Imaging. 2008;33:417-424.
8. Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn’s disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721-1727.
9. Yoon K, Chang KT, Lee HJ. MRI for Crohn's Disease: Present and Future. Biomed Res Int. 2015:786802.
10. Fidler JL, Guimaraes L, Einstein DM. MR imaging of the small bowel. Radiographics. 2009; 29:1811–1825.
11. Al-Hawary MM, Zimmermann EM, and Hussain HK. MR imaging of the small bowel in crohn disease. Magnetic Resonance Imaging Clinics of North America. 2014;22:13–22.
12. Seo N, Park SH, Kim KJ, et al. MR Enterography for the Evaluation of Small-Bowel Inflammation in Crohn Disease by Using Diffusion-weighted Imaging without Intravenous Contrast Material: A Prospective Noninferiority Study. Radiology. 2016;278:762-772.
13. Tantawy, HI, Algebally AMW. Role of Quantitative MRI Diffusion-Weighted Imaging (DWI) in detecting lesion activity in patients with Crohn’s Disease. The Egyptian Journal of Radiology and Nuclear Medicine. 2016;47:53-59.
14. Guhaniyogi S, Chu ML, Chang HC, et al. Motion immune diffusion imaging using augmented MUSE (AMUSE) for High-Resolution multi-shot EPI. Magn Reson Med. 2016;75:639-652.
15. Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995; 34:65–73.
16. Farzaneh F, Riederer SJ, Pelc NJ. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn Reson Med. 1990;14:123–139.
17. Miller KL, Pauly JM. Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med. 2003;50:343–353.
18. Chen NK, Guidon A, Chang HC, et al. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage. 2013;72:41–47.
19. Stocker D, Manoliu A, Becker AS, et al. Image quality and geometric distortion of modern Diffusion-Weighted Imaging sequences in magnetic resonance imaging of the prostate. Invest Radiol. 2018 53:200-206.
20. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine. 2016;15:155–163.
Figures