0809
Transfer Learning-Based Preoperative Prediction of Lymph Node Metastasis1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Radiology, Stony Brook University, Stony Brook, NY, United States, 3Psychiatry, Stony Brook University, Stony Brook, NY, United States
Synopsis
A tool to preoperatively predict sentinel lymph node status in patients with breast cancer could minimize the need for invasive surgical examination. Radiomics has been shown to have predictive power in many classification tasks. Fully automated deep learning methods would integrate more easily into clinical workflow because they do not require manual feature extraction. However, convolutional neural networks are computationally demanding and require large datasets to train. Transfer learning can be applied to allow for shortened training time and applicable to relatively small datasets.
Introduction
Development of a non-invasive preoperative predictive model as an alternative to sentinel lymph node biopsy (SLNB) is an active area of research which could minimize the need for invasive surgical examination of the axilla. This could spare many individuals the risk of side effects associated with surgical procedure, such as lymphedema or infection[1]. Radiomics and machine learning have potential to identify features that are not appreciable to the naked eye and has been shown to be useful in many classification tasks[2-4]. In this study, we modify and apply a deep learning-based model for prediction and compare it to conventional radiomics pipeline. A known disadvantage of radiomics analysis is the requirement of user-defined feature extraction. Deep learning-based models omit user intervention for feature extraction and would integrate easier into clinical workflow; however, they are known to be computationally demanding and require a large dataset to train. This study utilizes transfer learning, which applies knowledge learned from a previous task to a new task, allowing for shortened training time and applicable to relatively small datasets.Methods
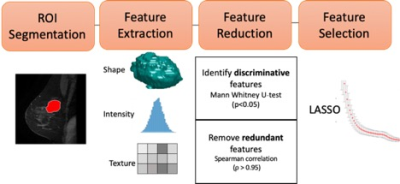
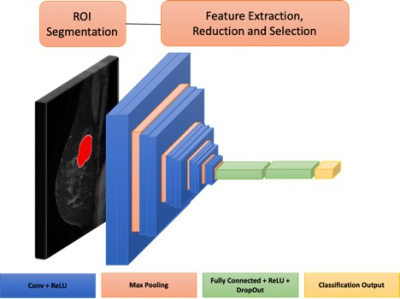
163 eligible patients with breast cancer undergoing primary surgery were retrospectively reviewed and randomly divided into two groups: train (n=109, 37 positive SLNB) and test (n=54, 18 positive SLNB). Wash-In, Wash-Out and Signal Enhancement Ratio maps were calculated from the dynamic contrast enhanced series of the preoperative MRI. Two methods were employed for prediction of sentinel lymph node status and are shown in Figures 1 and 2. 1) Radiomic features (shape, histogram, texture) were extracted and feature selection was performed using least absolute shrinkage and selection operator (LASSO), which is a conventional radiomics approach. 2) An adaptation of multilayer pre-trained convolutional neural network (CNN) VGG16[5]with unfreezing of last convolutional block and modification of classification layers to suit our binary prediction task. Both models utilized only imaging data and predictive performance was measured by receiver operator curve (ROC) analysis. To assess reproducibility of the models, LASSO and CNN were repeated with 100 random seeds.Results
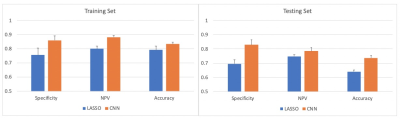
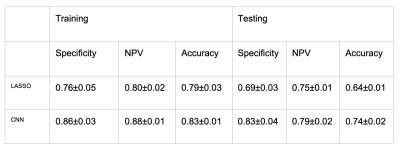
Results are summarized in Figures 3 and 4. Accuracy of the training and testing set was higher for CNN compared to LASSO (83% vs 79% and 74% vs 64%, respectively). Additionally, specificity and negative predictive value was higher in both the training and testing sets for CNN compared to LASSO (86% vs 76% and 83% vs 69%, respectively, 88% vs 80% and 79% vs 75%, respectively).Discussion
In the case of preoperative prediction of sentinel lymph node biopsy, in addition to accuracy, high specificity (true negative rate) is required to avoid the risk of under-treatment by not identifying metastatic disease. This study shows the ability of transfer learning framework to predict sentinel lymph node status using maps derived from standard-of-care MRI sequences with good accuracy and specificity with a relatively small dataset and omitting the requirement for handcrafted features (i.e., conventional radiomic analysis). Additionally, this study shows that the deep learning-based model is more generalizable to the testing set compared to the radiomics-based model.Conclusion
Transfer deep learning-based prediction has the potential to serve as a useful tool in the identification of diagnostic biomarkers from medical imaging for prediction of sentinel lymph node metastasis.Acknowledgements
This work is in part funded by National Institutes of Health (R03CA223052), Walk-for-Beauty Foundation and Carol M. Baldwin Breast Cancer Research Foundation.References
1. Hack, T.F., et al., Physical and psychological morbidity after axillary lymph node dissection for breast cancer.J Clin Oncol, 1999. 17(1): p. 143-9.
2. Rizzo, S., et al., Radiomics: the facts and the challenges of image analysis.Eur Radiol Exp, 2018. 2(1): p. 36.
3. Aerts, H.J.W.L., et al., Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach (vol 5, pg 4006, 2014).Nature Communications, 2014. 5.
4. Aerts, H.J., The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review.JAMA Oncol, 2016. 2(12): p. 1636-1642.
5. Simonyan, K. and A. Zisserman, Very deep convolutional networks for large-scale image recognition.arXiv preprint arXiv:1409.1556, 2014.
Figures