0808
Age estimation from whole-body MR images: A proof-of-principle study1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Medical Image and Data Analysis (MIDAS), University Hospital Tübingen, Tübingen, Germany, 3Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 4Department of Radiology, University Hospital Tübingen, Tübingen, Germany
Synopsis
Age is one of the most important clinical parameters describing patients in a medical context. The chronological age (CA) does however not necessarily reflect the true underlying biological age (BA) which can depend on multiple factors such as lifestyle, social environment, medical history, genetics and ethnicity. It is therefore desirable to measure BA quantitatively and objectively. In this proof-of-principle study, we examine if CA can be estimated from whole-body MRI. We propose a novel deep learning architecture to perform an accurate CA estimation.
Introduction
Each human ages differently depending on multiple factors such as lifestyle, social environment, medical history, genetics and ethnicity. Age is one of the most important parameters describing individuals in a medical context. In many clinical conditions, characteristic age distributions define a-priori probabilities for initial staging, therapy selection, drug administration and treatment monitoring1-3.However, the chronological age (CA) does not necessarily coincide with the actual biological age (BA) of the human body or of one specific organ. In fact, due to these age-related effects the general condition and specific capacities of individuals can deviate significantly from the average of the respective age group. These observations have motivated the concept of BA in contrast to CA4 and hence BA can be viewed as a measure of deviation from the age group. Despite of this vague definition of BA, the potential benefits for a personalized treatment are easily conceivable. However, the question remains on how to measure BA quantitatively and objectively.
Different age measures have been proposed in the past based on genetic, cellular, phenotypic and epidemiologic approaches4-10. In addition, clinical parameters such as functional parameters (e.g. lung function, cardiac function, blood pressure) or anthropometric measures (e.g. gender, body mass index, fat distribution) have been included8,10 in these studies. In principle, the use of medical imaging can be expected to provide substantial information. The assessment of age with MRI was mainly limited to skeletal9,11 and neurological10,12-14 applications. Manually extracted morphological features were used15 with machine learning classifications16,17. More recently, convolutional neural networks (CNNs) have been applied to predict the brain age from 2D MR images18,19 or from the whole volume with shallow 3D CNNs14,20.
None of the previous works focused on age estimation in a whole-body setting. Before an organ-specific BA can be estimated from whole-body MR images, it is required to accurately estimate the CA. In this work, we investigate the hypothesis that: CA is highly correlated with the image content. Moreover to be unbiased by any organ-specific features, the age estimation is done blind, i.e. on the whole-body T1w image without organ information. A CNN is proposed which performs a regression based on multiple 2D image orientations from the whole-body 3D MR and on anthropometric features. Influence of training database size and anthropometric features is investigated and the proposed network is compared against a CNN used for brain age estimation (deep Gaussian processes; DPG)13.
Methods
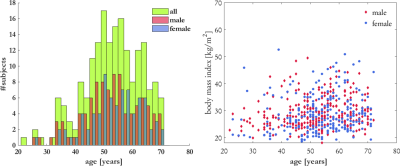
A hybrid 3D CNN is proposed for CA estimation which uses as input three orthogonal 2D images in axial, coronal and sagittal view of a multi breath-hold 3D T1w DIXON sequence (native axial, TE=1.23/2.46ms, TR=4.36ms, resolution=1.4x1.4x3mm, matrix size=320x260x316). Data was acquired in a multi-center epidemiological cohort study (German National Cohort; NAKO)21 on a 3T MRI. 200 subjects were randomly selected to uniformly represent age and gender as depicted in Fig.1. The training database consists of 157 subjects and 43 subjects are used for testing. Central slices of each orientation are normalized and taken as input (Fig.2).A blind CA estimation is investigated, i.e. no explicit attention focusing or pre-segmented organs are used, in order to test the hypothesis that CA is correlated to the image content, which is the pre-cursor for BA determination. Similar to previous works, we incorporate epidemiological parameters such as gender, height and weight of the subject into the network prediction.
The proposed AgeNet is depicted in Fig.3 and is composed of three parallel branches with a 2D CNN feature extractor and a squeeze-and-excitation22 for attention focusing. The outputs are then combined by a global average pooling and concatenated together with the epidemiological parameters to be further processed in three fully-connected layers with CA regression output. The network is trained end-to-end with Adam optimizer and mean absolute error (MAE) loss for 100 epochs and learning rate 10-4.
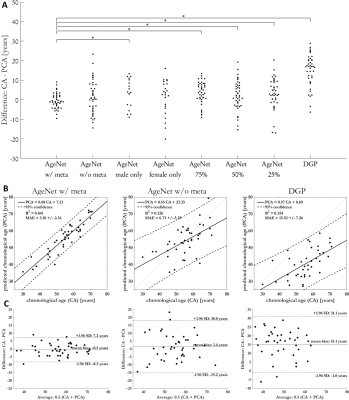
Difference between CA and predicted CA (PCA) are compared for various training scenarios (see Fig.4) and against a network for brain age estimation (DGP)13. Statistical significance was evaluated with a paired Welch’s t-test ($$$P<0.05$$$) and Bonferroni correction.
Results and Discussion
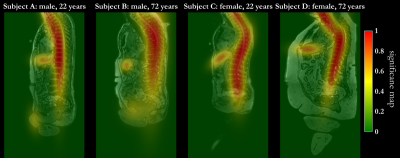
Fig.4 shows the comparison between the proposed AgeNet with and without epidemiological parameters, trained only on male/female subjects and using a smaller training database of 75%, 50% and 25%. AgeNet performs statistically significant better than all other approaches (except female only) (Fig.4a) and providing smallest MAE=3.2±2.3years (Fig.4b), minimal bias=-0.5years and tightest confidence intervals=±7.8years (Fig.4c). Without epidemiological parameters, the estimation is still feasible, but with larger uncertainty. Thus, epidemiological parameters help to focus the network’s attention. The DPG network fails to predict a reliable CA. Inspecting the Grad-CAM23 backpropagations in Fig.5, the network focused its decision making mainly on larger organs (liver) and the spine. This motivates thus the extension in the future to concentrate on an organ-orientated CA estimation from which subsequently a BA can be derived.This study has limitations. No organ-specific estimation was conducted. Due to limited training database, only a multi-slice 2D processing was feasible. Moreover, some age groups are currently underrepresented in this database.
Conclusion
CA estimation from whole-body MRI is feasible with a reliable estimation of MAE=3.2 years.Acknowledgements
No acknowledgement found.References
1. Niccoli T,
Partridge L. Ageing as a risk factor for disease. Current biology
2012;22(17):R741-R752.
2. Repetto L.
Greater risks of chemotherapy toxicity in elderly patients with cancer. The
journal of supportive oncology 2003;1(4 Suppl 2):18-24.
3. Story DA.
Postoperative complications in elderly patients and their significance for
long-term prognosis. Current Opinion in Anesthesiology 2008;21(3):375-379.
4. Jia L, Zhang W,
Chen X. Common methods of biological age estimation. Clinical interventions in
aging 2017;12:759.
5. Chen BH, Marioni
RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai P-C, Roetker NS, Just AC,
Demerath EW, Guan W. DNA methylation-based measures of biological age:
meta-analysis predicting time to death. Aging (Albany NY) 2016;8(9):1844.
6. Ignjatovic V, Lai
C, Summerhayes R, Mathesius U, Tawfilis S, Perugini MA, Monagle P. Age-related
differences in plasma proteins: how plasma proteins change from neonates to adults.
PloS one 2011;6(2):e17213.
7. Jylhävä J,
Pedersen NL, Hägg S. Biological age predictors. EBioMedicine 2017;21:29-36.
8. Nakamura E, Miyao
K. A method for identifying biomarkers of aging and constructing an index of
biological age in humans. The Journals of Gerontology Series A: Biological
Sciences and Medical Sciences 2007;62(10):1096-1105.
9. Albrecht E,
Sillanpää E, Karrasch S, Alves AC, Codd V, Hovatta I, Buxton JL, Nelson CP,
Broer L, Hägg S. Telomere length in circulating leukocytes is associated with
lung function and disease. European Respiratory Journal 2014;43(4):983-992.
10. Park J, Cho B,
Kwon H, Lee C. Developing a biological age assessment equation using principal
component analysis and clinical biomarkers of aging in Korean men. Archives of
gerontology and geriatrics 2009;49(1):7-12.
11. Mughal AM, Hassan
N, Ahmed A. Bone age assessment methods: A critical review. Pakistan journal of
medical sciences 2014;30(1):211.
12. Cole JH.
Neuroimaging-derived brain-age: an ageing biomarker? Aging (Albany NY)
2017;9(8):1861.
13. Popescu SG, Cole
JH, Glocker B, Sharp DJ. Deep Learning Methods for Estimating “Brain Age”from
Structural MRI Scans. Medical Imaging
with Deep Learning (MIDL); 2018.
14. Cole JH, Poudel
RP, Tsagkrasoulis D, Caan MW, Steves C, Spector TD, Montana G. Predicting brain
age with deep learning from raw imaging data results in a reliable and
heritable biomarker. NeuroImage 2017;163:115-124.
15. Wang J, Li W, Miao
W, Dai D, Hua J, He H. Age estimation using cortical surface pattern combining
thickness with curvatures. Medical & biological engineering & computing
2014;52(4):331-341.
16. Franke K, Ziegler
G, Klöppel S, Gaser C, Initiative AsDN. Estimating the age of healthy subjects
from T1-weighted MRI scans using kernel methods: exploring the influence of
various parameters. Neuroimage 2010;50(3):883-892.
17. Lao Z, Shen D, Xue
Z, Karacali B, Resnick SM, Davatzikos C. Morphological classification of brains
via high-dimensional shape transformations and machine learning methods.
Neuroimage 2004;21(1):46-57.
18. Huang T-W, Chen
H-T, Fujimoto R, Ito K, Wu K, Sato K, Taki Y, Fukuda H, Aoki T. Age estimation
from brain MRI images using deep learning.
IEEE International Symposium on Biomedical Imaging (ISBI); 2017. IEEE. p
849-852.
19. Ito K, Fujimoto R,
Huang T-W, Chen H-T, Wu K, Sato K, Taki Y, Fukuda H, Aoki T. Performance
Evaluation of Age Estimation from T1-Weighted Images Using Brain Local Features
and CNN. IEEE Engineering in Medicine
and Biology Society (EMBC); 2018. IEEE. p 694-697.
20. Ueda M, Ito K, Wu
K, Sato K, Taki Y, Fukuda H, Aoki T. An Age Estimation Method Using 3D-CNN From
Brain MRI Images. IEEE International
Symposium on Biomedical Imaging (ISBI); 2019. IEEE. p 380-383.
21. Bamberg F, Kauczor
H-U, Weckbach S, Schlett CL, Forsting M, Ladd SC, Greiser KH, Weber M-A,
Schulz-Menger J, Niendorf T. Whole-body MR imaging in the German National
Cohort: rationale, design, and technical background. Radiology
2015;277(1):206-220.
22. Hu J, Shen L, Sun
G. Squeeze-and-excitation networks. IEEE
conference on computer vision and pattern recognition (CVPR); 2018. p
7132-7141.
23. Selvaraju
RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-cam: Visual
explanations from deep networks via gradient-based localization. IEEE International Conference on Computer
Vision (ICCV); 2017. p 618-626.
Figures