0804
Multiple Brain Age Metrics Reveal Premature Brain Aging Network and Association with Clinical Factors in Schizophrenia1Institute of Medical Device and Imaging, College of Medicine, National Taiwan University, Taipei, Taiwan, 2Department of Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan, 3AcroViz Technology Inc., Taipei, Taiwan, 4Department of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan, 5Molecular Imaging Center, National Taiwan University, Taipei, Taiwan
Synopsis
It is unclear how brain regions contribute to the premature aging in schizophrenia and whether different brain age metrics would reveal distinct clinical relevance. Therefore, we developed multiple bias-free brain age metrics based on volumetric and microstructural information to quantify the brain aging of patients with schizophrenia. The results showed that the cortical areas and fiber tracts located in the prefrontal, temporal, and limbic regions manifested dominantly to the premature brain aging compared to the other areas. Also, white matter brain age showed the significant correlation with age of onset, medication dose, and negative symptom, manifesting better clinical sensitivity.
Introduction
The studies using brain age approach have reported that schizophrenia contributes to the premature aging process in human brains[1]. Multiple imaging modalities have been utilized to investigate this aberrant aging on gray and white matters (GM and WM)[2], however, it is unclear how those regions contribute to the premature brain age in schizophrenia and whether brain age metrics might reveal distinct clinical relevance. To address these issues, we developed multiple brain age metrics (including GM, WM, and the combined brain ages) based on T1-weighted images and diffusion spectrum imaging (DSI) datasets using machine learning approach. We estimated the degree of premature aging by transforming the conventional brain age into the normalized predicted age difference (nPAD) scores, which was an analog of PAD without being contaminated by age-related bias[3]. Higher nPAD score indicates more severe degree of premature aging. Next, incorporating with the region-specific normative model approach to quantify the local impairment in schizophrenia[4], the derived standardized scores (i.e., z-scores) from normative models were analyzed to construct the maximum correlation with nPAD score by canonical correlation analysis (CCA). The derived coefficients of z-scores in CCA represent the contribution of local impaired regions to the premature brain age metrics. We also investigated the association between nPAD scores and the clinical factors to explore the clinical relevance.Methods
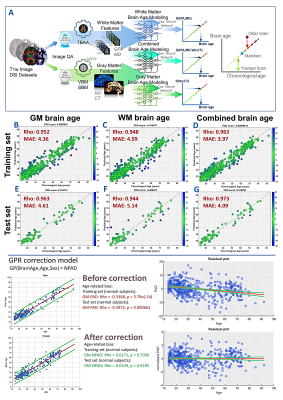
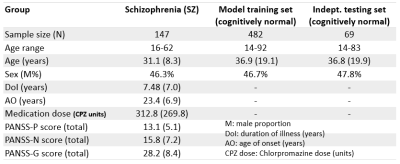
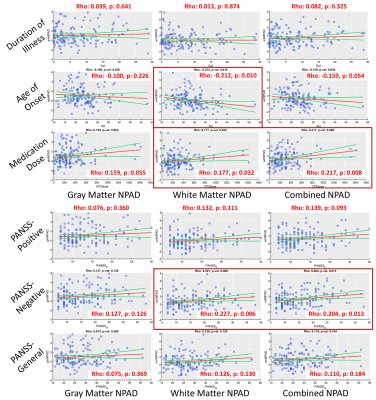
The multiple brain age metrics including GM, WM, and the combined brain ages were developed based on cerebral T1-weighted images and DSI datasets (Figure1-upper). The training and test sets used to establish brain age models and test model performance contained 482 and 69 healthy subjects, respectively (Figure2). Also, we enrolled the patients with schizophrenia (n=147) to conduct the experiment (Figure2). All images were acquired on a 3T MRI system (TIM Trio, Siemens). The imaging parameters were the same as the previous study[3]. All images were first checked by the quality assurance procedures. The T1w images were further analyzed by voxel-based and surface-based morphometries to estimate the volume and cortical thickness of GM, then transform into the region-specific features according to the LPBA-40 and Freesurfer-DK40 atlases[5]. Besides, the DSI datasets were reconstructed by the regularized version of diffusion MAP-MRI framework into generalized fractional anisotropy (GFA) and mean diffusivity (MD)[6]. The Whole-brain tract-specific analysis was conducted to sample the WM features according to the predefined 45 tracts from each diffusion index[7]. Three cascade neural network brain age models were established using the training set based on GM, WM, and the combined features, respectively (Figure1). Pearson’s correlation and mean absolute error were used to evaluate model performance. To correct the age-related bias, which attributed to the significant correlation of PAD with chronological age in the training set, we established Gaussian process regression (GPR) correction models in the training set to normalize the brain age metrics into the nPAD scores based on the given age and sex level (Figure1-lower). Finally, the prediction and correction models were applied to the patients to estimate the nPAD scores. Next, to explore which altered regions highly contributed to the premature brain age, we quantified the regional alterations by using normative models to transform feature indices into the z-scores, which represented the region deviation against the normal population. The CCA was conducted to correlate the patients’ nPAD scores with their z-scores. The derived coefficients of z-scores were normalized into [0,1] as the weights. The higher weights denoted the larger feature importance of brain regions contributing to the premature brain age. Also, correlation analyses were conducted to calculate the correlation of nPAD with the duration of illness, age of onset, medication dose, and symptom severity.Results
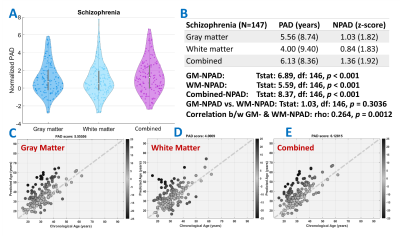
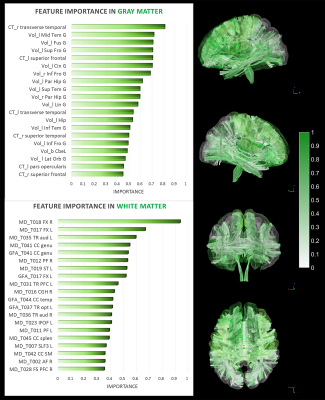
The model performance was satisfactory in both the training and test sets (Fig.1-middle). Also, the age-related bias was significantly alleviated by the correction models (Figure1-lower). The nPAD scores of schizophrenia in three brain age metrics, especially the combined metric, showed significant deviation from the norm, whereas no significant difference between nPADs of GM and WM (Figure3). In the results of CCA, the most important features in GM and WM were shown in Figure4. The volume and MD averagely contributed higher than the cortical thickness and GFA in the GM and WM, respectively. The cortical areas and fiber tracts located in the prefrontal, temporal, and limbic regions manifested dominantly to the premature brain aging. In the clinical relevance, the WM but not GM brain age metric showed significantly positive correlations with medication dose and negative correlation with age of onset. All brain age metrics did not significantly correlate with duration of illness. Moreover, the WM and combined brain age metrics revealed significant positive correlations with the severity of negative symptom.Discussion and Conclusion
Patients with schizophrenia exhibited significantly premature brain age in all metrics compared to the norm. Also, the high contribution of disease-affected brain regions to the brain age metrics provided the direct evidence of impaired regions linked to the premature aging in schizophrenia. That premature brain age had no association with the duration of illness was supported by the “early hit non-progressive” hypothesis[2]. Besides, WM brain age had better sensitivity to many clinical factors. This study unveiled the underpinning of premature aging network in schizophrenia and provided a neuroimaging reference for clinical prognosis.Acknowledgements
No acknowledgement found.References
[1] Kaufmann, Tobias, et al. "Common brain disorders are associated with heritable patterns of apparent aging of the brain." Nature Neuroscience 22.10 (2019): 1617-1623.
[2] Shahab, Saba, et al. "Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls." Neuropsychopharmacology 44.5 (2019): 898.
[3] Chen, Chang-Le, et al. "Premature white matter aging in patients with right mesial temporal lobe epilepsy: A machine learning approach based on diffusion MRI data." NeuroImage: Clinical (2019): 102033.
[4] Marquand, Andre F., et al. "Conceptualizing mental disorders as deviations from normative functioning." Molecular Psychiatry 24.10 (2019): 1415-1424.
[5] Christian Gaser and Robert Dahnke. “A Computational Anatomy Toolbox (CAT) for SPM” http://www.neuro.uni-jena.de/cat/index.html#VBM
[6] Hsu, Y.C., Tseng, W.Y. (2018) An efficient regularization method for diffusion MAP-MRI estimation. 2018 ISMRM-ESMRMB Joint Annual Meeting.
[7] Chen, Y.J., Lo, Y.C., Hsu, Y.C., Fan, C.C., Hwang, T.J., Liu, C.M., Chien, Y.L., Hsieh, M.H., Liu, C.C., Hwu, H.G., Tseng, W.Y. (2015) Automatic whole brain tract-based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Hum Brain Mapp, 36:3441-58.
Figures