0802
Altered resting-state functional activity characterizes white matter function in schizophrenia patients with long-term illness courses1Sichuan University, Chengdu, China
Synopsis
Increasing evidence has suggested white matter (WM) as well raised functional activity in brain and its functional information could be detected by resting-state functional MRI (rs-fMRI). Our study provided new insight into WM functional alterations over the long-term course of schizophrenia with and without the potential effects of antipsychotic medication. Functional changes in the splenium of the corpus callosum (SCC) were found in both treated and untreated patients, which may represent core WM functional changes in schizophrenia.
Introduction
Schizophrenia is one of the most severe psychiatric disorders and has a lifetime morbid risk of 1%, but the causes and pathogenesis remain poorly understood1. Recently, schizophrenia has been characterized by disconnected functional or structural neural networks2. Functional MRI studies have revealed hypoconnectivity among brain regions within the prefrontal cortex3. Structural studies of WM integrity revealed by diffusion tensor imaging (DTI) have indicated WM structural alterations predominantly in the corpus callosum (CC)4. The function of the white matter (WM) has rarely been studied due to limited proper methods before, but increasing evidence has begun to shed light on the existence to map WM activation and connectivity5,6. Here, we aim to unveil how WM function manifest itself in long-term untreated schizophrenia patients by rs-fMRI, which will be of great interest in providing novel information about WM dysfunction in schizophrenia.Methods
This study was approved by the local ethical committee and written informed consent was obtained from all subjects. A total of 91 subjects (Han Chinese) were recruited for current study, including 25 never-treated chronic schizophrenia patients, 41 patients who had taken long-term antipsychotic medications and 25 matched healthy controls. Rs-fMRI and T1-weighted images (T1WI) were collected on a 3T scanner (EXCITE, GE Healthcare) with an 8-channel phase array head coil. Amplitude of low frequency fluctuations (ALFF) in WM and the correlation coefficients between white and gray matter (GM) were examined and compared among the three participant groups by analysis of covariance (ANCOVA), with age and sex included as covariates. In addition, using independent component analysis (ICA) via GIFT software (version 3.0b. http://icatb.sourceforge.net/), rs-fMRI data in the WM were de-noised and decomposed into 20 spatially independent components, thus the WM functional organization were further evaluated. Correlational analyses were conducted to examine the relationship between ALFF values in regions with group difference and demographic and clinical variables, including age, PANSS scores and daily antipsychotic dose in chlorpromazine equivalents7.Results
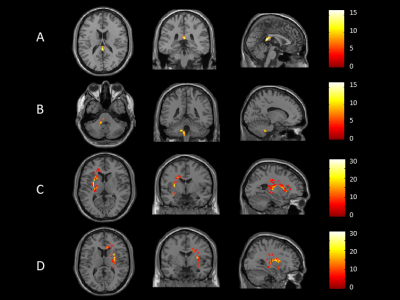
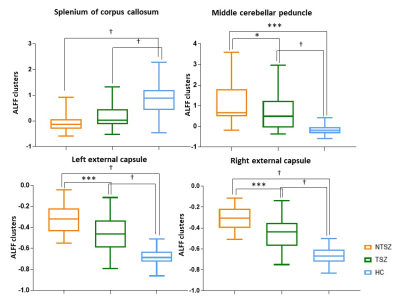
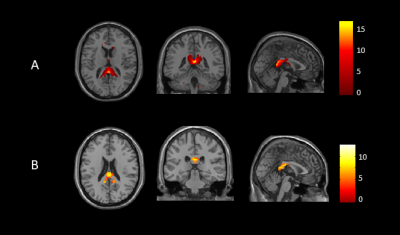
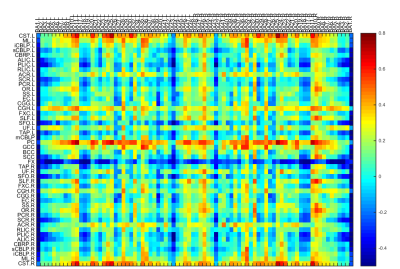
After pairwise comparisons of the three participant groups, we found that ALFF was significantly lower in the splenium of corpus callosum (SCC), and higher in the middle cerebellar peduncle (MCP) and bilateral external capsule (EC) in both never-treated patients and treated patients when compared to healthy controls, and greater in never-treated schizophrenia (Figure 1,2, p<0.05, FDR corrected). The ICA also showed significantly decreased functional connectivity in SCC in never-treated patients relative to treated patients or healthy controls (Figure 3, p<0.05, FDR corrected). The correlation coefficients of WM-GM showed significantly lower synchronization in the genu of corpus callosum (GCC), pontine crossing tract (PC), bilateral cingulum (hippocampus) (CGH) and bilateral corticospinal tract (CST) in treated patients relative to healthy controls (Figure 4, p<0.05, FDR corrected). Within the overall group of schizophrenia patients, PANSS scores were negatively associated with ALFF values in the SCC (r=-0.326, p<0.05; r=-0.267, p<0.05). No significant age-related associations were observed with ALFF changes using linear or quadric modeling in any patient subgroup. No significant correlation was observed between daily antipsychotic doses and ALFF values in the treated schizophrenia patients.Discussion
We studied WM function in a rare sample of long-term ill schizophrenia patients with and without antipsychotic treatment. The most important finding of the current study is the abnormal functional connectivity of SCC in long-term patients with schizophrenia. While the greater change within WM in never-treated patients was consistent with microstructural alterations observed in a DTI study4, which also suggested that long-term antipsychotic treatment may have a facilitative or neuroprotective effect on WM function via direct or indirect mechanisms over the long-term course of illness. Furthermore, based on the finding that the CC plays a notable role in maintaining stable functional communication between hemispheres8, we speculated that the dysfunction of the SCC may disrupted synchronization of WM and GM in treated schizophrenia patients. As one study9 showed that compensatory activation for the loss of functional integration in patients with schizophrenia, we speculated that the defective function of the SCC may be compensated by higher intracerebral activation of both sensorimotor regions. Our findings showed that only treated patients demonstrated abnormal synchronization of WM-GM, while no difference was observed between never-treated patients and healthy controls. Another WM functional network study also displayed a disrupted association between WM and GM networks in treated schizophrenia patients10. This abnormality may be due to the cumulative medication exposure along chronic course of illness. In addition, a strong link between symptoms and dysfunction of WM displayed a consensus between WM function and clinical manifestations.Conclusion
The present study revealed more widespread alterations in WM function in never-treated long-term schizophrenia patients than in those who received long-term antipsychotic treatment. Our findings may provide a novel view of WM functional alterations in never-treated and treated schizophrenia patients, and suggest that long-term antipsychotic treatment may also provide some benefits for WM function over the course of illness. Furthermore, the results demonstrated that lower ALFF in SCC in treated patients with schizophrenia may disrupt contact between bilateral cerebrum and disturb the synchronization of WM and GM. These findings raise the possibility of using WM function to uncover the coordinated function between WM and GM to elucidate how the brain works in schizophrenia patients.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Project Nos. 81671664 and 81621003), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project No. ZYYC08001). The authors declare no competing interests.References
1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (London, England). 2016;388:86-97.
2. Zhang J, Cheng W, Liu ZW, et al. Neural, Electrophysiological and Anatomical Basis of Brain-Network Variability and Its Characteristic Changes in Mental Disorders. Brain. 2016;139:2307-2321.
3. Lui S, Yao L, Xiao Y, et al. Resting-State Brain Function in Schizophrenia and Psychotic Bipolar Probands and Their First-Degree Relatives. Psychol Med. 2015;45:97-108.
4. Xiao Y, Sun H, Shi S, et al. White Matter Abnormalities in Never-Treated Patients with Long-Term Schizophrenia. Am J Psychiatry. 2018;175:1129-1136.
5. Gawryluk JR, Mazerolle EL, D'Arcy RC. Does Functional Mri Detect Activation in White Matter? A Review of Emerging Evidence, Issues, and Future Directions. Front Neurosci. 2014;8:239.
6. Li J, Biswal BB, Wang P, et al. Exploring the Functional Connectome in White Matter. Hum Brain Mapp. 2019;10.1002/hbm.24705.
7. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International Consensus Study of Antipsychotic Dosing. Am J Psychiat. 2010;167:686-693.
8. Shen K, Misic B, Cipollini BN, et al. Stable Long-Range Interhemispheric Coordination Is Supported by Direct Anatomical Projections. P Natl Acad Sci USA. 2015;112:6473-6478.
9. Tan HY, Sust S, Buckholtz JW, et al. Dysfunctional Prefrontal Regional Specialization and Compensation in Schizophrenia. Am J Psychiat. 2006;163:1969-1977.
10. Jiang Y, Luo C, Li X, et al. White-Matter Functional Networks Changes in Patients with Schizophrenia. Neuroimage. 2019;190:172-181.
Figures