0801
Altered functional synchrony between grey and white matter as a novel indicator of brain system dysconnectvity in schizophrenia1Sichuan University, Chengdu, China, 2University of Münster, Münster, Germany
Synopsis
Previous studies of white matter (WM) had been limited to structural assessment. In this study, by utilizing resting-state fMRI, the BOLD signals in WM and its functional correlations with BOLD signals in grey matter (GM) were assessed between antipsychotic-naive schizophrenia patients and healthy comparisons. The functional correlation coefficient defined as GM-WM functional synchrony were found widespread altered in patients, especially in WM connecting hemispheres, fronto-temporal, cortico-subcortical regions, and in prefrontal, cingulate, visual, temporal cortex. Additionally, age and illness-duration related alternations in functional synchrony shared the same trend. These findings described schizophrenia as a progressive disorder which was characterized by dysconnectivity.
INTRODUCTION
Schizophrenia was thought a progressive disorder1. With its distinct disease mechanisms remaining poorly understood, altered integrity between grey matter (GM) and white matter (WM) had been proposed to be a possible factor in the pathophysiology of schizophrenia2. Although controversy over whether WM functional activity could robustly give rise to BOLD signals had not been concluded, BOLD signals were reported to be observed in WM with resting-state fMRI in recent study3. Thus, by investigating BOLD signals in WM and its functional correlations with BOLD signals in GM on antipsychotic-naive schizophrenia patients and healthy comparisons, this study provided additional assessment of connectivity disorders such as schizophrenia and intact functional connectivity in human brain. Particularly, we were interested in whether age would show different associations with GM-WM functional correlation between diagnostic groups.METHODS
This study included 78 antipsychotic-naive schizophrenia patients with illness duration ranging from 0.2 months to 37 years and 102 healthy comparisons. While healthy comparisons had a higher level of education relative to controls, age and gender were comparable between two groups. All resting-state fMRI and T1-weighted images were collected on the same 3-T scanner (EXCITE, GE, Milwaukee). The image preprocessing was carried out using Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm) and the analysis pipeline was in accordance with that of a previous study4. A mean time series was constructed by averaging BOLD signals across GM regions and WM tracts according to atlas in both groups separately. Then, it was used to yield the correlation analysis between pairwise GM and WM. All data were analyzed using SPSS (Statistical Product and Service Solutions for Windows version 24.0, https://www.ibm.com/analytics/spss-statistics-software). Group differences in correlation coefficients were tested using analyses of covariance (ANCOVA) with age as covariate. Linear regression analysis was performed to assess the potential influence of age and illness-duration on correlation coefficients respectively.RESULTS
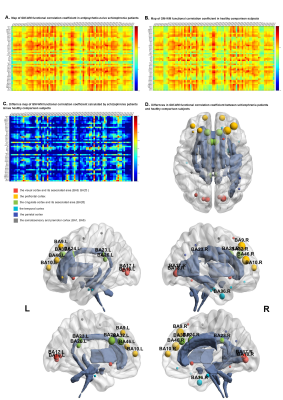
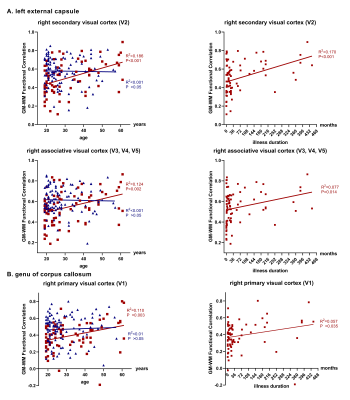
Compared to healthy comparisons, schizophrenia patients showed weaker GM-WM functional correlations in widespread regions (p<0.05, Bonferroni corrected), especially in genu of corpus callosum, bilateral uncinate fasciculus, bilateral cingulum, bilateral cerebral peduncle, pontine crossing tract,left external capsule with visual cortex, prefrontal cortex, cingulate cortex, temporal cortex. More specifically, schizophrenia patients showed a positive linear relationship between age and GM-WM correlation coefficients mainly in left external capsule, genu of corpus callosum with visual cortex (p<0.01, uncorrected). When utilizing illness duration as a solo covariate, a positive linear relationship was also showed with GM-WM correlation coefficients.DISCUSSION
To our knowledge, this is the first study to demonstrate synchrony alterations of functional correlations between GM and WM, which was related to a broader brain network in schizophrenia patients independently from possible confounds by medication. More importantly, age-related alternations in GM-WM synchronously functional correlation were observed for the first time in schizophrenia patients. We suggested a broader network of weaker GM-WM functional correlations in patients compared to controls especially in white matter tracts connecting hemispheres5, fronto-temporal, cortico-subcortical regions. This widespread disruption or dysconnectvity might be interpreted as a consequence of compensation mechanisms of surrounding increased GM and WM networks6.Notably, though the functional correlations in WM disrupted extensively, theirs corresponding GM distributions were relatively concentrated. This might suggest the ‘long-range communications’ between GM and WM were mainly disrupted in schizophrenia patients. Supported by the opinion that the anatomical and functional connectivity was dissociated7 in schizophrenia patients, this findings suggested investigating the functional activity in WM tracts rather than speculating it based on the inaccurately spatial coherence from DTI studies was irreplaceable for the intact human connectivity. Since whether WM altered at first or not was uncertain8,findings of this study might be a novel predictable indicator of dysconnectivity in schizophrenia without having to distinguish the primary alternation.
More importantly, strong associations between GM-WM correlation coefficient and age were found in this study. The positive association between age and GM-WM functional correlation suggested a progressive disruption and an increased communication took place among visual cortex, left external capsule and genu of corpus callosum. The GM-WM functional correlation coefficient of schizophrenia patients exceeded the healthy comparison subjects beyond the age of 45s which might be the decompensation of increased activation in visual cortex. While hallucination was not directly associated with age9, it was reported to occur more frequently in chronic patients. Notably, schizophrenia patients who didn’t undergo hallucination during first-episode were at low risk of hallucination with the course of illness. This might bring clinical attention to a timely treatment and the urge for short durations of untreated psychosis (DUPs) before mechanisms of compensation may be no longer sufficient, especially in developing country, where schizophrenia patients tend to receive insufficient antipsychotic treatment.
CONCLUSION
The findings from the present study extend the knowledge about neurobiological brain system alterations related to schizophrenia independent from antipsychotic medication by using a novel approach to study BOLD signal changes in WM and GM-WM functional correlations. Our findings support the model of dysconnectivity in widespread brain systems as a major neuropathological change of schizophrenia being reflected by disruptions of synchronously functional activation between grey matter and white matter. In addition, the findings of this study emphasized the relevance of age-related alternations in functional correlations thereby giving further support to a model of schizophrenia as a progressive disease.Acknowledgements
References
1. Diethelm, O. 1973. Dementia Praecox and Paraphrenia - Kraepelin,E. American Journal of Psychiatry, 130(10): 1167-1168.
2. Moskowitz, A., & Heim, G. 2011. Eugen Bleuler's Dementia praecox or the group of schizophrenias (1911): a centenary appreciation and reconsideration. Schizophr Bull, 37(3): 471-479.
3. Marussich, L., Lu, K. H., Wen, H. G., & Liu, Z. M. 2017. Mapping white-matter functional organization at rest and during naturalistic visual perception. Neuroimage, 146: 1128-1141.
4. Ding, Z. H., Huang, Y. L., Bailey, S. K., et al 2018. Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proceedings of the National Academy of Sciences of the United States of America, 115(3): 595-600
5. Mori, S., Oishi, K., Jiang, H. Y., et al. J. 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage, 40(2): 570-582
6. Jiang, Y. C., Luo, C., Li, X., et al. 2019. White-matter functional networks changes in patients with schizophrenia. Neuroimage, 190: 172-181.
7. Skudlarski, P., Jagannathan, K., Anderson, K., Stevens, M. C., Calhoun, V. D., Skudlarska, B. A., & Pearlson, G. 2010. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry, 68(1): 61-69.
8. Fornito, A., Zalesky, A., Pantelis, C., & Bullmore, E. T. 2012. Schizophrenia, neuroimaging and connectomics. Neuroimage, 62(4): 2296-2314.
9. Clark, M. L., Waters, F., Vatskalis, T. M., & Jablensky, A. 2017. On the interconnectedness and prognostic value of visual and auditory hallucinations in first-episode psychosis. European Psychiatry, 41: 122-128
Figures