0800
A Triple Network Connectivity Study in patients with first episode schizophrenia1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Alzheimer's Disease Research Network, Hong Kong, Hong Kong, 3State Key Laboratory of Brain and Cognitive Sciences, Hong Kong, Hong Kong, 4Castle Peak Hospital, Hong Kong, Hong Kong, 5Neuropsychology and Applied Cognitive Neuroscience Laboratory, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China, 6Department of Psychology, University of Chinese Academy of Sciences, Beijing, China, 7Department of Psychiatry, The University of Hong Kong, Hong Kong, Hong Kong, 8Philips Healthcare, Hong Kong, Hong Kong
Synopsis
To investigate the major psychopathology of first-episode schizophrenia (SCZ), the triple network model consisting of central executive network (CEN), salience network (SN) and default mode network (DMN) was employed. Group-level independent component analysis and group comparison between schizophrenia patients and healthy subjects within networks were applied. In the results, the SCZ group presented significant hyperconnectivity in bilateral insula within SN and hypoconnectivity in occipital lobe and medial prefrontal cortex within DMN. In addition to that, connectivity in bilateral insula within SN showed significant correlation with PANSS scores.
Introduction
The abnormalities of multiple resting state networks have been detected in patients suffering from schizophrenia (SCZ)1, 2. A triple network model which consists of central executive network (CEN), salience network (SN) and default mode network (DMN) has been applied to investigate the dysfunctions of networks in schizophrenia.3 However, studies have got inconsistent results and many of them failed to shed light on the core psychopathology of schizophrenia. To understand more about this disease, our study evaluated the intrinsic resting functional connectivity of the triple network model in patients and healthy control (HC) using independent component analysis (ICA), and correlation of clinical profiles with the network abnormalities.Methodology
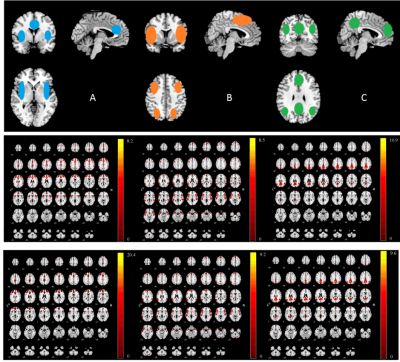
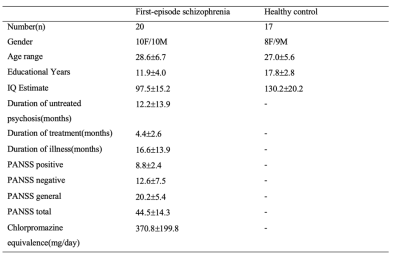
Twenty first-episode schizophrenia subjects and seventeen healthy controls participated in the study. Positive and Negative Syndrome Scale (PNASS) was used to measure the severity of symptoms of patients. (Table 1) All participants were scanned with a Phlips-3T MR scanner (Achieva TX, Philips Healthcare, Best, The Netherlands) using a standard 8 channel head coil. Structural images were acquired with 3D fast field echo sequence (3D-T1-FFE sagittal, TR=7ms, TE=3.2ms, Flip angle=8°, voxel size=1×1×1mm3, FOV=240×240×160mm3). Functional images were collected by using a gradient-echo-planar sequence (parameters: TR=2000ms, TE=30ms, flip angle=90°, voxel size=3×3×4mm3) sensitive to blood-oxygen-level-dependent (BOLD) contrast. For the resting-state fMRI, subjects were instructed to open their eyes and view the central fixation cross without thinking of anything in particular. After the preprocess of the fMRI data (DPABI, http://rfmri.org/dpabi), the analysis of data was performed using Group ICA Of fMRI Toolbox (GIFT). SN, CEN and DMN were identified from 25 group-level spatial intrinsic connectivity maps. Two sample t test was used to calculate the significance between two groups (FDR<0.05, cluster size>270mm3). The relationships between clinical features and peak value of network functional connectivity were estimated based on the Pearson correlation method in SPSS package (SPSS Inc., Chicage, USA).Results
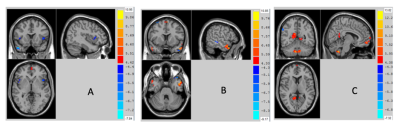
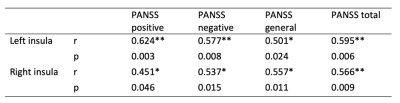
Figure 1 showed the independent components (ICs) that best matched the SN, CEN and DMN for both the first episode schizophrenia and healthy groups. Compared with healthy group, significant increase was found in bilateral insula within SN and statistical decrease could be seen in medial prefrontal cortex (MPFC) and occipital lobe within DMN in SCZ group (Figure 2). There was no region of interest found in the group comparison of CEN. Significant correlation was found between PANSS scores and hyperconnectivity in bilateral insula (Table 2), but not between PANSS with hypoconnectivity in occipital and MPFC.Discussion and conclusion
The primary role of SN was to modulate the switching between the internally self-related cognition (DMN) and external oriented attention (task-based activity)4. There might be a link between the decrease of DMN connectivity with visual symptoms and increase of SN with emotional disturbances. Further investigation will be needed for such relationships. Also, the hyperconnectivity in insula seems to be an important feature of first episode schizophrenia with clinical positive and negative symptoms, which could be characterised as a biomarker of SCZ.Acknowledgements
No acknowledgement found.References
1. Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia Bulletin. 2014;40:428-437
2. Palaniyappan L. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry & Neuroscience. 2012;37:17-27
3. Wu X, Li Q, Yu X, Chen K, Fleisher AS, Guo X, et al. A triple network connectivity study of large-scale brain systems in cognitively normal apoe4 carriers. Frontiers in Aging Neuroscience. 2016;8
4. Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr Res. 2010;117:1-12
5. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569-12574
Figures