0799
Triple Network Hypothesis-related disrupted connections are associated with positive and negative symptoms in first-episode schizophrenia1The Fourth Military Medical University,Xi’an,China, Xi’an, China, 2MR Research China,GE Healthcare, Beijing, China
Synopsis
Schizophrenia
is one complex mental disorder. However
the dysregulated cross-network interactions among the SN, CEN and
DMN and how they contributed to different symptoms is still not clear. By
analyzing network interactions among the SN, CEN and DMN in patients
and controls
using DCM,as
well as the relationship
between network dynamics and clinical symptoms,
our
study provides strong evidence for the dysregulation among SN, CEN
and DMN in a triple-network perspective in first-episode schizophrenia. We
further proved that the connection between DMN and CEN could be
clinically-relevant neurobiological signature of schizophrenia symptoms.
INTRODUCTION
Schizophrenia is one highly disabling psychiatric disorder characterized by a range of positive and negative symptoms. The neurobiology characteristic in the brain from a network perspective is still poorly understood, leading to a lack of potential biologically-based markers and limited therapeutic efficacy. In the current study, we aimed to investigate resting-state effective connectivity among Central Executive Network (CEN), Default Mode Network (DMN) and Salience Network (SN) in first-episode Schizophrenia patients and healthy controls using dynamic causal modeling (DCM), which could quantify the strength of connections and confirm the flow direction from one region to another as one powerful tool for the effective connectivity analysis. We assumed that the disorganized directed communication among the three networks could be bio-marker for schizophrenia symptoms. To be more specific, we hypothesized that (i) the SN-centered motivational overall supervision was inhibited in schizophrenia; (ii) the balanced interaction between CEN and DMN were aberrant and the anticorrelated effect might be SN centered or directed; (iii) the different direction of interaction among networks could be associated with different symptoms in SZ.Methods:
The study sample consisted of 76 first-episode SZ patients and 80 age and sex-matched healthy controls (HCs) recruited by advertisement from the local community. All participants gave written informed consent approved by the local Research Ethics Committee after a complete description of this study. The fMRI were acquired on a 3.0-Tesla MRI scanner (GE Healthcare, Milwaukee, WI, USA). Resting state functional scans were acquired with an echo planar imaging (EPI) sequence using the following parameters: repetition time = 2000ms, echo time = 30ms, flip angle = 90°, field of view = 240 × 240mm, matrix = 64 × 64, slice thickness = 3.5mm, number of slices = 45. After pre-processing, resting-state fMRI images were decomposed into spatially independent spatial maps using the GIFT toolbox (http://mialab.mrn.org/software/gift/index.html). A general linear model (GLM) then was constructed for each participant. Eleven regions of interest (ROIs) including the left precunesus (PreC), left frontal superior medial cortex (MFC), left angular (LPC) and right angular (RPC) from DMN, right angular (RPG), left angular (LPG), left middle frontal cortex (LFG) and right middle frontal (RFG) from CEN, anterior cingulate cortex (ACC), left middle frontal cortex (lmFG) and right middle frontal (rmFG) from SN were selected. We examined network interactions among the SN, CEN and DMN in first-episode schizophrenia vs. matched controls using DCM. Further analysis of the relation between network dynamics and clinical positive and negative symptoms were performed.Results:
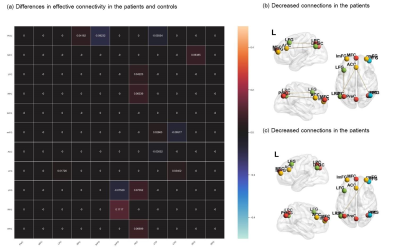
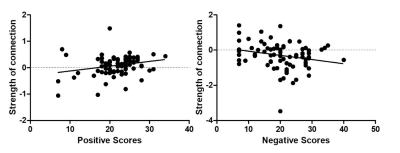
We observed that the DMN, CEN and SN across healthy controls and patients with schizophrenia showed several similarities within or between-network pattern in the resting state (Figure 1). Compared schizophrenia to controls, increased self-connections within the DMN and CEN subnetworks were observed (Figure 2). SN-centered cross-network interactions were mostly significantly reduced including the connections from SN to CEN and SN to DMN subnetwork 2 (Figure 2). Crucially, the strength of connections from DMN subnetwork 1 to CEN subnetwork 1 was associated positively to the Positive Score of PANSS (Figure 3). The connection from the CEN subnetwork 2 to DMN subnetwork 2 was negatively correlated with the Negative Score of PANSS (Figure 3).Discussion
Consistent with our hypothesis we found the dynamic functional interactions in patients with schizophrenia. First, SN-centered functional interactions were observed. Notably, the connections from SN to CEN and partial DMN showed significantly reduced interactions in first-episode schizophrenia patients. Further, the exiting of the connections between CEN and DMN were proved and was subnetwork different. Moreover, the psychosis symptoms in schizophrenia were correlated with certain connections in schizophrenia patients. In schizophrenia, the suppression of DMN by CEN was associated with the severity of negative symptoms, pointing the way toward corroborating core psychopathology. Our findings suggest that the dynamic functional interactions of the three networks are impaired in schizophrenia. Large-scale brain network models are now increasingly being used to study psychopathology and analysis of large-scale networks has shown to be powerful tools for investigating the core features of disorders for schizophrenia. We use the specific large-scale brain network model in investigate dynamic functional circuits in schizophrenia and the relationship to symptoms. We proved DMN subnetwork 1 to CEN network1, was associated positively to the Positive Score of PANSS. And the CEN network 2 to DMN subnetwork 2 was correlated negatively with the Negative Score of PANSS. However, we did not observe the SN-centered network pattern with severity of symptoms. We assumed that although SN attributed to the switching between CEN and DMN related to the external and internal stimuli 32, the existing of DMN and CEN interactions could lead to different symptoms, as increased self-connections within the DMN and CEN networks was observed. In conclusion, we investigated large-scale brain organization focuses on a ‘triple network model’, which highlights the role of the three above-mentioned networks that play distinct roles in human cognition and internal mental processes. Our findings also emphasize the over-suppression of brain network, a putative neural circuit for symptoms in schizophrenia. From a graph-theoretical point of view, we can expect network-derived metrics to facilitate identification of symptom heterogeneity and eventually aid in development of more targeted pharmacological and cognitive interventions.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants number 81571651, 81601474), Key Research and Development Program of Shaanxi Province (Grant number 2017ZDXM-SF-048).References
1. Stephan KE, Friston KJ and Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia bulletin. 2009; 35: 509-27.
2. Uddin LQ, Supekar KS, Ryali S and Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011; 31: 18578-89.
3. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007; 27: 2349-56.
4. Fox MD and Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007; 8: 700-11.
5. Raichle ME. The brain's default mode network. Annual review of neuroscience. 2015; 38: 433-47. 6. Chen Q, Chen X, He X, Wang L, Wang K and Qiu B. Aberrant structural and functional connectivity in the salience network and central executive network circuit in schizophrenia. Neuroscience letters. 2016; 627: 178-84.
7. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia bulletin. 2014; 40: 428-37.
8. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences. 2011; 15: 483-506.
9. Wang X, Zhang W, Sun Y, Hu M and Chen A. Aberrant intra-salience network dynamic functional connectivity impairs large-scale network interactions in schizophrenia. Neuropsychologia. 2016; 93: 262-70.
10. Menon V and Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010; 214: 655-67.
Figures