0798
Pretreatment white matter integrity predicts one-year clinical outcome in first episode schizophrenia1West China Hospital of Sichuan University, Chengdu, China, 2Sun Yat-sen University Cancer Center, Guangzhou, China, 3The First People’s Hospital of Ziyang, Ziyang, China
Synopsis
The unrevealing neuropathology underlying different clinical outcome has blocked effective treatment of schizophrenia. In this study, by prospectively recruited patients at baseline and followed them up for one-year, we have revealed the promising role of disrupted white matter integrity in discriminating good outcome from poor outcome schizophrenia. Further, the baseline white matter integrity in left anterior thalamus radiation is positively correlative with reduction of clinical ratings after one-year in all the patients. These findings indicated the underlying substrates in patients with different clinical outcomes and can serve as the potential imaging characteristic in differentiating these patients before initiating of antipsychotics.
Introduction
Schizophrenia is characterized by disrupted domains of cognition, thought and emotion and affects nearly 1% of the world’s population.1 Antipsychotics remain the mainstay treatment of schizophrenia, but almost 1/3 of patients show limited response to antipsychotics.2 Research into the pretreatment brain features associated with clinical outcome is needed to identify patients less likely to respond to first-line treatments who may need more intensive behavioral treatments or clozapine.3, 4Methods
A total of 56 first-episode drug-naive schizophrenia patients and 69 healthy controls were recruited for this study at baseline and were followed up after one year antipsychotic medication treatment. Clinical outcome for schizophrenia patients was assessed using Positive And Negative Syndrome Scale (PANSS) score decline according to the following formula:$$ (PANSS_baselinescore-PANSS_endpointscore ) /(PANSS_endpointscore-30)$$5, with good outcome patients identified based on a ≥50% decline of PANSS score while poor outcome patients showing less than a 50% decline of PANSS scores. The MRI scans of all participants were performed on a GE Signa EXCITE 3.0-T scanner (GE Healthcare) with an 8-channel phase array head coil. DTI data were preprocessed using FDT toolbox (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) followed by tract based spatial statistics (TBSS), which yield skeletonized images for fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD). The statistical analysis for image data was carried out with voxel-wise analysis on the skeletonized data using permutation-based non-parametric testing (randomized 5000 times) among the three groups and the significant p-value with the family-wise error (FEW) cluster corrected threshold was set at p< 0.05 with a cluster size over 100 voxels. Besides, correlation analysis was carried out between the abnormal diffusion tensor measures of white matter tracts and reduction of PANSS scores after treatment within the whole patient group. The receiver operating characteristic (ROC) curve analysis was then conducted using the significant different white matter tracts to assess the discriminating role in separating patients of different clinical outcomes.Results
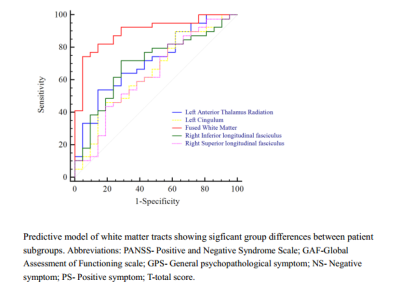
Significant differences were found in FA and RD in long fiber tracts that connect frontal, temporal, occipital, subcortical and cortical regions. All the patients showed reduced FA and increased RD compared with healthy controls. There was no significant difference in AD and RD among the three groups. Of note, compared with good outcome patients, poor outcome patients showed increased FA in right superior longitudinal fasciculus and right inferior longitudinal fasciculus, and decreased FA in left cingulum and left anterior thalamic radiation (Figure 1). There was no significant difference between poor outcome patients and good outcome patients in AD, MD or RD. Baseline FA in anterior thalamic radiation was positively correlated to reduction of PANSS scores (r=0.398, p=0.003). The combination of altered white matter FA showed promising capability in discriminating poor outcome patients from good outcome patients (sensitivity=74.4%, specificity=95.2%, AUC=0.90, p<0.0001), while FA in right inferior longitudinal fasciculus (sensitivity=71.8%, specificity=71.4%, AUC=0.70, p=0.005) and anterior thalamic radiation (sensitivity=53.85%, specificity=85.7%, AUC=0.72, p=0.0017) showed moderated differentiating performance (Figure 2).Discussion
One of the most interesting finding is that although good outcome and poor outcome schizophrenia patients showed similar reduced FA and increased RD in white matter tracts at baseline, the two patient subgroups differed in FA in left cingulum, left anterior thalamic radiation, right superior longitudinal fasciculus and right inferior longitudinal fasciculus. Although previous findings of schizophrenia patients have revealed abnormal white matter integrity in these fiber tracts that may underpin the neuropathological changes in this disorder6-8, limited information was provided with regard to discriminating patients with distinct clinical outcomes. Most previous studies treated schizophrenia as a homogenous disorder and findings were based on the gross differences between patients and healthy controls. While schizophrenia is heterogeneous in clinical manifestations and clinical outcomes. Brain metabolism studies have revealed dopamine synthesis capacity abnormality in poor outcome schizophrenia but not in good outcome patients.9 Our significant findings of ROC analysis results provided potential insights into separating patients with different clinical outcomes. Although previous studies have found altered brain structure or function between good outcome and poor outcome schizophrenia patients, most studies remained a descriptive comparison and lacked the discriminating utility in separating the two subgroups in practice. Another interesting finding worth noting is that a better performance of integrated characteristics was observed over single measure in differentiating responders and non-responders with ROC analysis. This finding was in accordance with the widespread rather than localized white matter alterations in schizophrenia, as both brain functional or structural studies have shown widespread altered brain measures in first episode drug-naïve schizophrenia, and these abnormalities lied mainly in frontal, temporal and subcortical areas and always presented together. 10, 11 Therefore, the combination of disrupted white matter provided comprehensive and complementary information in differentiating patients with different clinical outcomes. Although this is a preliminary study of separating different clinical outcome patients at baseline, further studies are needed on early detection of different clinical outcome schizophrenia and targeted treatment to improve patient prognosis.Conclusion
Our findings provided potential imaging characteristic in differentiating patients of distinct clinical outcomes before initiating of antipsychotics. Future studies of individual patient identification may help to early detect patients for appropriate treatment and improve prognosis.Acknowledgements
No acknowledgement found.References
1. Dhindsa R S and Goldstein D B. Schizophrenia: From genetics to physiology at last. Nature. 2016;530: 162-3.
2. Mouchlianitis E, Bloomfield M A, Law V, et al. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr Bull. 2016;42: 744-52.
3. Meltzer H Y. Treatment-resistant schizophrenia--the role of clozapine. Curr Med Res Opin. 1997;14: 1-20.
4. Tarcijonas G and Sarpal D K. Neuroimaging markers of antipsychotic treatment response in schizophrenia: An overview of magnetic resonance imaging studies. Neurobiol Dis. 2019;131: 104209.
5. Leucht S, Davis J M, Engel R R, et al. Definitions of response and remission in schizophrenia: recommendations for their use and their presentation. Acta Psychiatr Scand Suppl. 2009;7-14.
6. Zeng B, Ardekani B A, Tang Y, et al. Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res. 2016;172: 1-8.
7. Rigucci S, Rossi-Espagnet C, Ferracuti S, et al. Anatomical substrates of cognitive and clinical dimensions in first episode schizophrenia. Acta Psychiatr Scand. 2013;128: 261-70.
8. Ebdrup B H, Raghava J M, Nielsen M O, et al. Frontal fasciculi and psychotic symptoms in antipsychotic-naive patients with schizophrenia before and after 6 weeks of selective dopamine D2/3 receptor blockade. J Psychiatry Neurosci. 2016;41: 133-41.
9. Demjaha A, Egerton A, Murray R M, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75: e11-3.
10. Zhang Y, Zheng J, Fan X, et al. Dysfunctional resting-state connectivities of brain regions with structural deficits in drug-naive first-episode schizophrenia adolescents. Schizophr Res. 2015;168: 353-9.
11. Ellison-Wright I, Glahn D C, Laird A R, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165: 1015-23.