0795
Relapse Risk Revealed by Degree Centrality and Cluster Analysis in Heroin Addicts Undergoing Methadone Maintenance Treatment1Department of Radiology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China, 2Department of nuclear medicine, Tangdu Hospital of Air Force Medical University of PLA, Xi'an, China
Synopsis
The objective of this study is to identify the heroin dependents undertaking stable methadone maintenance treatment (MMT patients) at high risk for opioid relapse prospectively. First, a self-defined addiction-related brain network was constructed with 10 hubs of several circuits associated with addiction and their degree centrality. Next, sixty male MMT patients was classified into different subgroups through grouping their addiction-related network into distinct neuronal activity patterns by K-means clustering algorithm. By comparing relapse rate between subgroups with distinct network pattern, the one at high risk for relapse was identified. This finding implicated a novel strategy for improving MMT therapeutic effect.
INTRODUCTION
Heroin addiction is a chronic disease characterized by compulsive drug seeking and use. In clinical practice, relapse to illegal drug use or dropout remain the biggest challenge faced by the heroin addicts undergoing methadone maintenance treatment (MMT)1. It is important to predict relapse risk in MMT patients for improving MMT outcomes. Neurobiological imaging researches have demonstrated that some brain hubs of neuronal circuits are essential for addiction behavioral and relapse2,3. This study aimed to identify the heroin dependents undertaking stable MMT at high risk for opioid relapse using clustering analysis prospectively, based on a self-defined addiction-related network made of those brain hubs and their degree centrality (DC). Data of brain regions contributed to relapse are also provided.METHODS
This study was approved by the Institution Board of the Fourth Military Medical University. Sixty male MMT patients( mean age, 35.8 years; age range, 22-53 years) and 29 matched healthy controls (HC) ( mean age, 34.8 years; age range, 19-48 years) were recruited in this study. MRI data were acquired with a 3.0 T GE-Signa HDxt MRI scanner using an eight-channel head coil (GE Healthcare, Milwaukee, U.S.A.). After brain resting-state functional MRI data acquisition,the monthly illegal drug use information of 60 MMT patients in a 26-month follow-up phase was obtained by a structured interview assessing and a urine drug test. According to the widely accepted neurobiological theory for addiction, 10 addiction-related hubs with their degree centrality (DC) were chosen to construct a user-defined addiction-related network for MMT patients. These regions included the bilateral nucleus accumbens (NAc), amygdala, anterior cingulate cortex (ACC), caudate, orbital frontal cortex (OFC), hippocampus, insular, putamen, thalamus, and dorsolateral prefrontal cortex (DLPFC). Weighted DC measures were calculated using the “REST-DC” toolkit in the REST V1.8 package3 for those regions mentioned above. After regressed out total methadone consumption, the DC value extracted from the 10 pairs of hubs of the 60 MMT patients was used to make a 60 x 20 matrix M, representing the addiction-related network. Then, the matrix M was classified into subgroups by clustering analysis of K-means clustering algorithm with R (https://www.R-project.org/). By comparing relapse rate between subgroups with distinct network pattern, the one at high risk for relapse was identified. The differences in DC between MMT subgroups and HC were conducted with two-sample t-test on the whole brain level. The difference in relapse rate between subgroups was calculated via Pearson's Chi-squared test with Yates' continuity correction. Finally, poisson regression analysis was used to investigate the brain regions had significant contribution to relapse.RESULTS
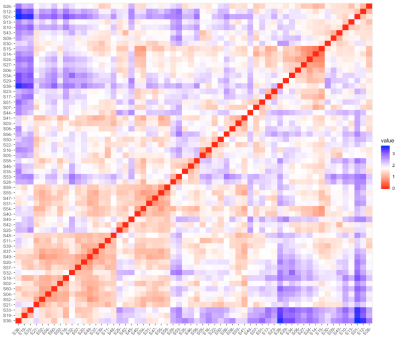
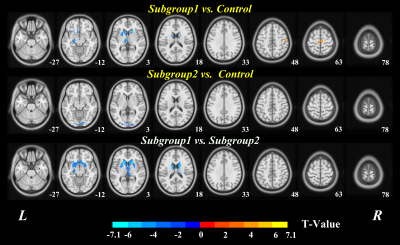
The 60 MMT patients were classified into 2 subgroups, with 29 MMT patients (48.3%) in subgroup1 and 31 (51.7%) in subgroup2, according to the addiction-related network patterns determined by the best clustering number K (Fig. 1). Except relapse rate and total heroin consumption (p<0.05), the two subgroups had no significant differences in demographic, psychological indicators and clinical information (p>0.05) (Fig.3). The subgroup with high-relapse rate (HRG) had wide range of DC changes in cortical-striatal-thalamic circuit and orbital frontal cortex (OFC) relative to the HC, while that of low-relapse subgroup (LHR) was limited (TFCE corrected p< 0.05 and cluster size K>10). Compared to LHR, GHR had reduced DC in mesocorticolimbic circuits, including bilateral amygdala, caudate, OFC, thalamus, hippocampus, nucleus accumbens (NAc), putamen, ventral anterior cingulate cortex (vACC), and left insular (TFCE corrected p< 0.05 and cluster size K>10) (Fig.2). DC of NAc, vACC, hippocampus and putamen were negatively correlated with MMT patients’ relapse rate respectively (p<0.001).DISCUSSION
This present study constructed addiction-related brain network for MMT patients, in which hubs and nodes come from mature addiction theories and the topological feature was described by DC. Then, the poor responders to treatment were prospectively identified by clustering the data set of MMT patients’ addiction-related network. It is noteworthy that no differences were found in demographic characteristics, methadone use or BDI and HAMA score between the high relapse subgroup and low relapse subgroup except total heroin consumption. The HRG had a broader area with decreased DC and more heroin consumption than LRG had. The significant differences of DC between HRG and LRG mainly located in mesocorticolimbic system. The finding that distinct MMT patients have different network pattern may reflect drug use associated brain alterations or distinct genetic influence4-8. Furthermore, The finding that DC value of NAc, vACC, hippocampus and putamen correlated with the MMT patients’ relapse rate hinted the essential role of these areas in maintaining addiction behavioral and potential role for addiction pharmacotherapy9-12. The high light of this study is that identification of MMT patients at high relapse risk allows for improved treatment tailoring, whereby healthcare providers can target more aggressive adjunct therapies within these high-risk populations.CONCLUSION
Two distinct addiction-related brain network patterns were discovered in MMT patients prospectively,while those having widespread DC abnormalities suffered higher risk for relapse . The finding that distinct MMT patients have different network pattern may reflect drug use associated brain alterations or genetic influence. This finding implicated a novel strategy for improving MMT therapeutic effect. The brain areas implicated playing an important role in relapse behavioral could be selected as pharmacotherapeutic targets for high-risk populations.Acknowledgements
No acknowledgement found.References
1 Volkow, N.D., et al., Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry, 2019. 76(2): p. 208-216.
2 Volkow, N.D., J.S. Fowler, and G.J. Wang, The addicted human brain: insights from imaging studies. J Clin Invest, 2003. 111(10): p. 1444-51.
3 Naqvi, N.H., et al., The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci, 2014. 1316: p. 53-70.
4 Wollman, S.C., et al., White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse, 2015. 41(2): p. 133-8.
5 Zhang, R., et al., Abnormal white matter structural networks characterize heroin-dependent individuals: a network analysis. Addict Biol, 2016. 21(3): p. 667-78.
6 Volkow, N.D., et al., Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A, 2011. 108(37): p. 15037-42.
7 Noble, E.P., Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur Psychiatry, 2000. 15(2): p. 79-89.
8 Nestler, E.J., Epigenetic mechanisms of drug addiction. Neuropharmacology, 2014. 76 Pt B: p. 259-68.
9 Li, N., et al., Nucleus accumbens surgery for addiction. World Neurosurg, 2013. 80(3-4): p. S28 e9-19.
10 Gabriele, A. and R.E. See, Lesions and reversible inactivation of the dorsolateral caudate-putamen impair cocaine-primed reinstatement to cocaine-seeking in rats. Brain Res, 2011. 1417: p. 27-35.
11 Wang, W., et al., Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chin Med J (Engl), 2010. 123(12): p. 1582-8.
12 Kaplan, G.B., et al., Opiate sensitization induces FosB/DeltaFosB expression in prefrontal cortical, striatal and amygdala brain regions. PLoS One, 2011. 6(8): p. e23574.
Figures