0793
Magnetic susceptibility, R2* and iron evolve during reperfusion injury wound healing1Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 4Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Surgery, Columbia University Irving Medical Center, New York City, NY, United States, 6Chemistry, Columbia University, New York City, NY, United States, 7Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 8Biochemistry and Molecular Biophysics Graduate Group, Perelman School of medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
There are multiple forms of iron including protein bound and labile iron found in reperfusion injury of acute myocardial infarction (MI). This study investigated iron accumulation, molecular form of iron, and cellular response to reperfusion injury with respect to the duration of wound-healing, in a large animal model. We demonstrate with magnetic susceptibility and R2* imaging biomarkers, there is a significant increase in infarct iron content in acute reperfusion injury that dissipates by 8-week post-MI and validate these findings with histology, iron concentration, and mRNA expression.
Purpose
Reperfusion injury is a complication seen in 40-60% acute myocardial infarction (MI) patients receiving primary percutaneous coronary intervention therapy1,2. While reperfusion substantially improves myocardial salvage, ischemia and microvascular damage can cause extravasation of RBCs and elevation in iron content including protein bound and labile (“free”) iron. Labile iron can cause reactive oxygen species through the Fenton/Haber–Weiss reaction cycle leading to cellular damage3,4. In previous studies, a paramagnetic shift in reperfusion injury at 1-week post-MI was associated with elevated iron content5. There remains limited understanding of the alteration of myocardial tissue and iron content throughout wound healing and the association to MRI biomarkers (magnetic susceptibility, R2*). The objective of this study was to evaluate the progression of wound healing through quantitative susceptibility mapping (QSM), R2*-mapping, histology, iron concentration and investigate the molecular form of iron and mRNA expression of cellular markers of iron homeostasis in reperfusion injury.Methods
MI was induced by coronary surgical ligation and released after 90- or 180-min in male Yorkshire swine. Ex vivo multi-echo gradient-echo image acquisition of whole heart specimens occurred at 3-day (n=2), 1-week (n=6) and 8-week (n=2) post-MI. Scan parameters include 7 T: 0.5 mm (Figure 1A,B), 0.75 mm (Figure 1C, 2C,D), 3 T: 0.9 mm (Figure 2A,B) isotropic resolution, TR/TEfirst/TElast/ΔTE=24,42/2.8,3.3/16.5,16.1/3.4,3.2 msec, FA=28,16 degrees, BW=725,610 Hz/pixel, NEX=3,1 (7 T parameters are bold italics). R2*-maps were obtained using a 2-parameter fit with least squares minimization. QSM was reconstructed using the MEDI image processing pipeline6,7. Two volumes-of-interest (VOIs) were obtained from T2*-weighted (T2*w) images using threshold active contour segmentation (ITK-SNAP8) including remote myocardium (“isointense”) and infarct (“hyperintense” and “hypointense”). Tissue experiments included histology staining (Prussian blue, Trichrome, H&E, Hemoglobin-beta immunohistochemistry (HBB IHC)), total iron concentration from inductively coupled plasma-optical emission spectrometry (ICP-OES), labile iron concentration from electron paramagnetic resonance (EPR) spectroscopy. Statistics included linear regressions and student’s t-test comparison between infarct and remote myocardium regions.Results
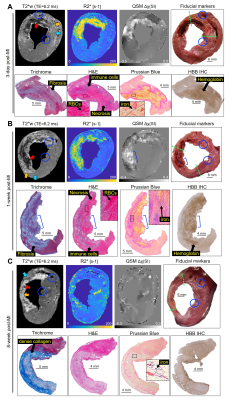
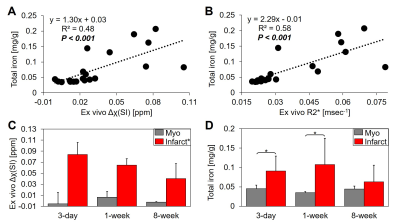
Figure 1 displays the ex vivo QSM and R2* results obtained from 90-min reperfused infarcts at 3-day (1A), 1-week (1B) and 8-week (1C) post-MI. Histology shows initial fibrosis (Trichrome) at 3-day that becomes dense collagen scar tissue by 8-week. At 3-day and 1-week lysed myocytes and RBCs are present with an active immune response at the transition zone of myocyte necrosis that extends toward the endocardium by 1-week suggesting infiltrative immune cells originating outside the infarct (H&E). By 1-week, regions containing ferric iron (Fe3+) were at the transition zone (Prussian blue) which could correspond to engulfment of iron by immune cells. Hemoglobin (HBB IHC), which could be paramagnetic, was present at 3-day and 1-week. By 8-week, RBCs, immune cells, iron and hemoglobin had dissipated.Figure 2 shows good linearity between total iron concentration vs. magnetic susceptibility (Δχ: slope=1.30 mg/g of total iron per 1 ppm increase in tissue magnetic susceptibility, R2=0.48, P<0.001) (2A) and total iron concentration vs. relaxation rate (R2*: slope=2.29 mg/g of total iron per 1 msec-1 increase in R2*, R2=0.58, P<0.001) (2B). Infarct magnetic susceptibility showed a paramagnetic shift relative to remote myocardium (0.06±0.02 vs. 0.001±0.01 ppm, P<0.001) (2C) corresponding to elevated infarct total iron concentration (0.09± 0.05 vs. 0.04±0.01 mg/g, P<0.001) (2D). Both infarct susceptibility and iron decreased by 8-week post-MI.
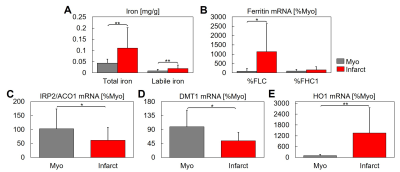
Figure 3 shows labile iron concentration, a fraction of total iron concentration measured, was significantly increased in infarct compared to remote regions (0.019± 0.016 vs. 0.008±0.007 mg/g, P<0.001) (3A). Ferritin light chain (FLC) expression was significantly increased in the infarct vs. remote myocardium (1137.2 vs. 88.1 %Myo, P=0.002), unlike ferritin heavy chain (FTH1) (P=0.15) (3B). The expression of the intracellular iron sensor Iron Regulatory Protein 2 (IRP2, also known as ACO1) was significantly decreased in the infarct region vs. remote myocardium (61.4 vs. 102.4 %Myo, P=0.03) (3C). Divalent metal transporter 1 (DMT1), which transports iron into the labile iron pool, was significantly decreased in the infarct region vs. remote myocardium (53.7 vs. 99.1 %Myo, P=0.002) (3D). Heme oxygenase-1 (HO1) transcription is activated in response to increased intake of heme proteins, and its expression was significantly increased in the infarct region vs. remote myocardium (1350.3 vs. 100.5 %Myo, P<0.001) (3E), which corresponds to elevated hemoglobin seen with HBB IHC (1A,B).
Discussion and Conclusion
Our analysis showed several aspects of iron accumulation and cellular handling in reperfusion injury, there was an elevation in total iron concentration and positive Prussian blue staining in acute reperfusion injury (3-day, 1-week) that dissipated by 8-week post-MI (chronic reperfusion injury). During homeostasis, most of the intracellular iron is bound to ferritin. A small fraction of intracellular iron belongs to the labile iron pool9 and is redox active with the potential of generating free radicals upon MI injury4,10. In high intracellular iron conditions such as MI, IRP2/ACO1 is down regulated, inhibiting DMT1 and increasing ferritin gene expression4. The expression of FLC was significantly increased in infarct regions, suggesting an effective cellular response towards labile iron sequestration11. Significantly increased HO1 within infarct regions suggests cellular clearance of extracellular hemoglobin, where HBB IHC showed hemoglobin cleared during wound healing by 8-week post-MI. The ionization state as well as the molecular form of protein-bound iron, is expected to change throughout wound healing, affecting the magnetic susceptibility.Acknowledgements
We gratefully acknowledge support from R00-HL108157, R01-HL137984, NRSA in interdisciplinary Cardiovascular Biology NIH T32-HL007954, and HHMI-NIBIB Interfaces Program NIH T32-EB009384.References
1. Carrick D, Haig C, Ahmed N et al. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ Cardiovasc Imaging. 2016;9(1).
2. Carrick D, Haig C, Ahmed N et al. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients After Acute ST-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J Am Heart Assoc. 2016;5(2).
3. Krause GS, Joyce KM, Nayini NR et al. Cardiac arrest and resuscitation: brain iron delocalization during reperfusion. Ann Emerg Med. 1985;14(11):1037-1043.
4. Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531(1-2):81-92.
5. Moon BF, Kamesh Iyer S, Solomon MP et al. Magnetic susceptibility of hemorrhagic myocardial infarction: correlation with tissue iron and comparison with relaxation time MRI. Paris, France: International Society for Magnetic Resonance in Medicine annual meeting; 2018.
6. Liu T, Khalidov I, de Rochefort L et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24(9):1129-1136.
7. Liu T, Liu J, de Rochefort L et al. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: comparison with COSMOS in human brain imaging. Magn Reson Med. 2011;66(3):777-783.
8. Yushkevich PA, Piven J, Hazlett HC et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116-1128.
9. Kakhlon O and Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med. 2002;33(8):1037-1046.
10. Chevion M, Jiang Y, Har-El R et al. Copper and iron are mobilized following myocardial ischemia: Possible predictive criteria for tissue injury. Proc. Natl. Acad. Sci. USA. 1993;90(3):1102-1106.
11. Sammarco MC, Ditch S, Banerjee A and Grabczyk E. Ferritin L and H subunits are differentially regulated on a post-transcriptional level. J Biol Chem. 2008;283(8):4578-4587.
Figures