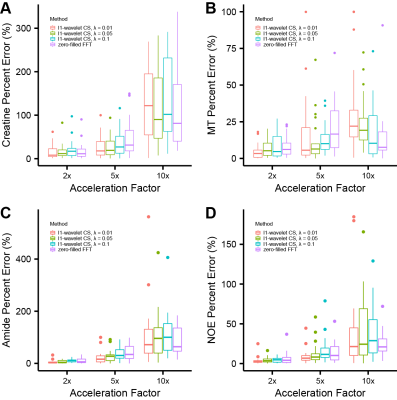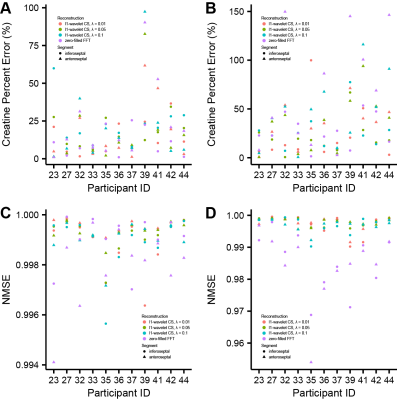0792
Effects of Accelerated Acquisition of Myocardial Creatine CEST MRI in the Healthy Human Heart at 3T1Bioengineering, University of California Berkeley, Berkeley, CA, United States
Synopsis
CEST-MRI is emerging as a powerful modality for molecular imaging of cardiac metabolites and fibrosis. In order to derive all contrasts (amide proton transfer: APT, creatine, and magnetization transfer: MT) a substantial number of differently CEST-weighted images must be acquired. Application of compressed sensing for cardiac CEST enables a 5x acceleration of acquisition with preserved accuracy.
Introduction
CEST-MRI is emerging as a powerful modality for molecular imaging of cardiac metabolites and fibrosis. In order to derive all contrasts (amide proton transfer: APT, creatine, and magnetization transfer: MT) a substantial number of differently CEST-weighted images must be acquired. This results in long scans with multiple breath-holds. Methods to accelerating acquisition while maintaining z-spectra fidelity, minimizing CEST contrast error, and recovering image quality can dramatically increase the likelihood of cardiac CEST-MRI adoption in clinical settings. Compressed sensing (CS) approaches leverage implicit sparsity in MRI to reconstruct from highly under-sampled k-space data. Although CS has been applied widely in the realm of real-time cardiac MR1, and CEST for high-grade glioma2, the application to cardiac CEST has not been examined. While small deviations in the signal intensity of CS-reconstructed images for anatomical imaging may prove inconsequential, for the derivation of CEST contrast such deviations may significantly impact the fidelity of derived CEST contrast. In this study we tested the impact of L1-wavelet CS reconstruction and acceleration rates on the subsequent error of cardiac CEST measurement of endogenous contrasts.Methods
Fully-sampled cardiac mid-ventricular short-axis CEST-MRI images at end-diastole were acquired from 11 healthy participants at offset frequencies from +10.0ppm to -10.0ppm in a 3T Siemens Trio MRI scanner. The cartesian fully sampled k-spaces were later under-sampled in the phase-encoding (PE) direction by 2x, 5x, and 10x acceleration factors, corresponding to 50%, 20%, and 10% of the original k-space. Several variable density randomly under-sampled masks were generated from a polynomial probability density function, leaving at least 10% of the fully sampled center PE lines as a calibration region. The masks for each acceleration factor were selected by measuring the wavelet transform point spread function (TPSF) for each mask, and choosing the one with the lowest peak interference, as previously described3. Images were then reconstructed using L1-wavelet CS using BART. Z-spectra were obtained for each participant in two regions of the myocardium, inferoseptal and anteroseptal, and Lorentzian fitted to 5 CEST pools (water, MT, NOE, creatine, and amide) using a Lorentzian difference method4.Results
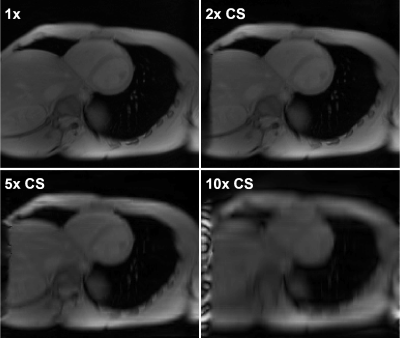
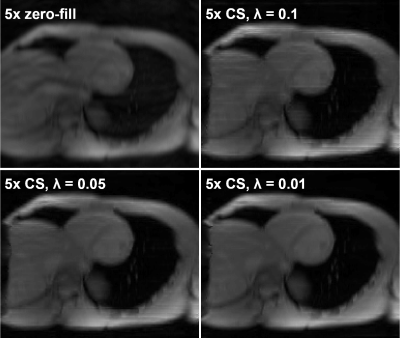
L1-wavelet CS reconstruction was able to recover the details and reduce incoherent aliasing artifacts of CEST-MRI acquisitions at different accelerations factors, with residual aliasing becoming more evident past 5x acceleration (Fig 1). An example of reconstruction at different λ is shown in figure 2. When comparing the Lorentzian fitted CEST contrast error for creatine, MT, amide, and NOE, CS reconstruction resulted in reduced error compared to zero-filled reconstruction at lower acceleration factors, but significant error at higher acceleration factors (Fig 3). The z-spectra for each participant at every acceleration factor and regularization parameter was compared to the corresponding fully sampled z-spectrum, and their robustness measured by normalized mean square error (NMSE). Figure 4 shows the NMSE for each CS method and the creatine percent error for each participant at two different acceleration factors, highlighting the impact of λ upon accuracy, and the increased z-spectra fidelity with CS compared to zero-filled FFT reconstruction.Discussion
CS reconstruction of cardiac CEST data is highly dependent upon selection of appropriate regularization parameter λ, which represents a unique trade-off between data fidelity and aliasing artifact removal for each participant. CS reconstruction was able to recover image quality at 5x acceleration factor, but the integrity of derived CEST contrasts began to suffer. Maintaining image quality for cardiac CEST-MRI is important since a unique myocardial mask must be drawn for each acquisition at a different offset frequency saturation. Participant motion between acquisitions, different breath-hold performance and heart rate variability for each acquisition result in slightly shifted myocardial position and shape between scans, requiring ROI-wise analysis in place of voxel-wise. Analysis of z-spectra by NMSE revealed that CS was able to maintain fidelity of the full z-spectra for most participants and methods, but details on signal attenuation by the CEST contribution of metabolites such as creatine were variable, resulting in larger than optimal error.Conclusion
CEST acquisition with fewer phase-encoding steps may reduce scan time through both shorter breath hold duration and potential packing of multiple offset images into a single breath hold. More work is needed to fine tune an optimal CS method to recover z-spectra with error within a range acceptable for a confident estimate of myocardial creatine content and distribution.Acknowledgements
The authors would like to thank Mark Velasquez for his assistance with scanning, and Dr. Jon Tamir for his insights on CS.References
1. Kido T, Kido T, Nakamura M, et al. Compressed sensing real-time cine cardiovascular magnetic resonance: accurate assessment of left ventricular function in a single-breath-hold. J Cardiovasc Magn Reson. 2016; 18:50.
2. Heo HY, Zhang Y, Leed DH, et al. Accelerating chemical exchange saturation transfer (CEST) MRI by combining compressed sensing and sensitivity encoding techniques. Magn Reson Med. 2017; 77(2):779-786
3. Lustig M, Donoho D, and Pauly J. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn Reson Med. 2007; 58(6):1182-1195.
4. Deshmane A, Zaiss M, Lindig T, et al. 3D gradient echo snapshot CEST MRI with low power saturation for human studies at 3T. Magn Reson Med. 2007; 81(4):2412-2423.
Figures