0789
Respiratory Motion-compensated High-resolution 3D Whole-heart T1ρ Mapping1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Cardiac T1ρ mapping has shown promising results for detecting ischemic cardiomyopathy without the need of exogenous contrast agents. Current 2D myocardial T1ρ mapping requires multiple breath-holds and provides limited coverage of the heart. In this study, we proposed a free-breathing 3D T1ρ mapping technique featuring whole heart coverage, near-isotropic spatial resolution (1.7×1.7×2mm3) and 100% respiratory acquisition efficiency. With the proposed technique, five T1ρ weightings were acquired in a clinically feasible scan time (~6 min), based on which 3D T1ρ maps were estimated. The accuracy and feasibility of the 3D technique was investigated in phantoms, healthy subjects and patient.
Introduction
Cardiac magnetic resonance T1ρ mapping has shown promising results for detecting ischemic cardiomyopathy without the need of exogenous contrast agents (1,2). Current 2D myocardial T1ρ mapping requires multiple breath-holds and provides limited coverage of the heart (3,4). Respiratory gating by diaphragmatic navigation has recently been exploited to enable free-breathing 3D T1ρ mapping, which, however, has low acquisition efficiency and may result in unpredictable and long scan times (5). This study aims to develop a fast respiratory motion-compensated 3D whole-heart myocardial T1ρ mapping technique with high spatial resolution and predictable scan time.Methods
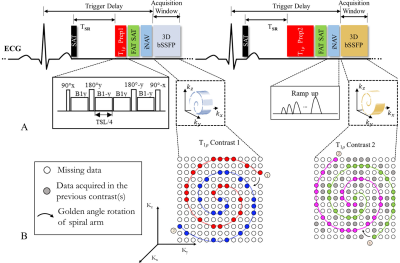
Sequence Design: The proposed 3D T1ρ mapping prototype sequence is performed under free-breathing with mid-diastolic ECG-triggering and consists of a non-selective saturation pulse (SAT), T1ρ preparation (T1ρ prep), fat suppression, 2D image navigator (iNAV) (6) and 3D balanced steady-state free-precession (bSSFP) readout (Fig. 1A). Five differently T1ρ-weighted volumes are acquired sequentially with increasing spin-lock time (TSL = 0, 10, 20, 35, 50 ms). To ensure the same magnetization before each T1ρ prep and the same cardiac motion state for the acquisition in each cardiac cycle, TSR and trigger delay (Fig. 1A) were fixed for all T1ρ preparations with different TSLs. In T1ρ prep (Fig.1A), besides the tip-down, tip-up pulses (90±x) and four separate spin-lock pulses with alternating phases (B1±y), 2 refocusing pulses with opposite phases (180±y) are added to make the T1ρ prep more robust to both B1 and B0 inhomogeneities. The B1 amplitude of spin-lock pulse is set to 400 Hz. A 3D variable-density Cartesian sampling with spiral-like profile order (VD-CASPR) (7) with an undersampling factor of 3.8 is employed. The spiral-like arm acquired in each heartbeat is rotated with golden-angle order (Fig. 1B) to ensure incoherent undersampling artifacts.Respiratory motion compensation and reconstruction: The respiratory motion correction comprises two steps: 2D beat-to-beat translational motion correction for each T1ρ-weighted dataset with 2D foot-head and left-right translational motion estimated from iNAVs (Fig. 2); 3D translational alignment between the different T1ρ-weighted volumes which are obtained by zero-filled reconstructions of the undersampled data (Fig. 2D). The motion corrected undersampled k-space is reconstructed using HD-PROST (8), which exploits local, non-local and contrast redundancies of the 3D multi-contrast images. After reconstruction, T1ρ map is obtained by pixel-wise fitting of the T1ρ-weighted images. The signal equation for the saturation recovery T1ρ prepared acquisition is given by:$$S=S_{0}\left(T_{SR},T_{1}\right)exp^{-TSL/T_{1\rho}}$$where $$$S$$$ is the signal intensity of the given T1ρ-weighted image, $$$S_{0}\left(T_{SR},T_{1}\right)$$$ is the signal before T1ρ prep, which is a saturation recovery function of TSR and T1.
MR imaging: Acquisitions were performed on a 1.5T scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen Germany). Phantoms with different T1ρ values were imaged with simulated heartrate of 60 bpm to test the accuracy of the accelerated 3D T1ρ mapping method in comparison with fully sampled 2D T1ρ mapping technique which acquired a single k-space line per heartbeat to reduce the influence of T1 relaxation during readout (3). For in vivo study, six healthy subjects and one patient were recruited after IRB approval and informed consent. The free-breathing 3D T1ρ mapping was acquired in coronal orientation with spatial resolution=1.7×1.7×2mm3, FOV=300×300mm2, 45-60 slices, TR/TE = 3.6/1.2 ms, flip angle = 50°, acquisition window≤126ms, scan time about 6 mins. For healthy subjects, the breath-hold 2D T1ρ mapping acquisitions were performed for comparison at mid short-axis location with spatial resolution=2×2mm2; slice thickness=8mm; TSL=0, 10, 20, 35, 50ms; breath-hold duration of about 20s. For the patient with suspected myocardial disease, breath-hold LGE scan (9) was performed.
Results
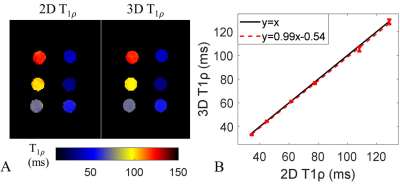
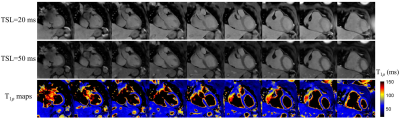
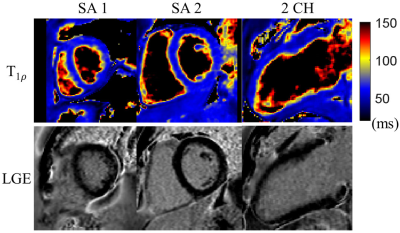
Phantom results (Fig. 3) indicate a good agreement of the 3D and 2D T1ρ mapping methods (R2=0.99). T1ρ maps were successfully reconstructed for all 7 subjects. Representative T1ρ-weighted images and T1ρ maps of one healthy subject with the proposed 3D free-breathing approach are shown in Fig. 4. Septal T1ρ values of the 6 healthy subjects measured with the 3D free-breathing approach were 59.0 ± 4.1ms, comparable to measurements with the breath-hold 2D method (58.3 ± 4.5ms, P>0.05). LGE images and reformatted 3D T1ρ maps of the patient are shown in Fig. 5. There is no myocardial enhancement on LGE and homogeneous myocardial T1ρ map was observed.Discussion
In this study, a novel free-breathing 3D whole heart T1ρ mapping technique was proposed, which enabled 100% respiratory scan efficiency and near-isotropic spatial resolution (1.7×1.7×2mm3) in a clinically feasible scan time of ~6mins, achieving similar accuracy to breath-hold 2D T1ρ mapping. Patients with myocardial infarction will be recruited to test its ability for non-contrast myocardial tissue characterization.Acknowledgements
No acknowledgement found.References
1. Muthupillai R, Flamm SD, Wilson JM, Pettigrew RI, Dixon WT. Acute myocardial infarction: Tissue characterization with T1(rho)-weighted MR imaging - Initial experience. Radiology 2004;232(2):606-610.
2. Witschey WRT, Zsido GA, Koomalsingh K, Kondo N, Minakawa M, Shuto T, McGarvey JR, Levack MM, Contijoch F, Pilla JJ, Gorman JH, Gorman RC. In vivo chronic myocardial infarction characterization by spin locked cardiovascular magnetic resonance. J Cardiovasc Magn R 2012;14.
3. Berisha S, Han J, Shahid M, Han YC, Witschey WRT. Measurement of Myocardial T-1 rho with a Motion Corrected, Parametric Mapping Sequence in Humans. Plos One 2016;11(3).
4. Wang L, Yuan J, Zhang SJ, Gao M, Wang YC, Wang YX, Ju SH. Myocardial T1rho mapping of patients with end-stage renal disease and its comparison with T1 mapping and T2 mapping: A feasibility and reproducibility study. J Magn Reson Imaging 2016;44(3):723-731.
5. Iyer SK, Moon B, Hwuang E, Han YC, Solomon M, Litt H, Witschey WR. Accelerated free-breathing 3D T1 cardiovascular magnetic resonance using multicoil compressed sensing. Journal of Cardiovascular Magnetic Resonance 2019;21.
6. Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012;67(2):437-445.
7. Prieto C, Doneva M, Usman M, Henningsson M, Greil G, Schaeffter T, Botnar RM. Highly Efficient Respiratory Motion Compensated Free-Breathing Coronary MRA Using Golden-Step Cartesian Acquisition. J Magn Reson Imaging 2015;41(3):738-746.
8. Bustin A, Lima da Cruz G, Jaubert O, Lopez K, Botnar RM, Prieto C. High-dimensionality undersampled patch-based reconstruction (HD-PROST) for accelerated multi-contrast MRI. Magn Reson Med 2019;81(6):3705-3719.
9. Holtackers RJ, Chiribiri A, Schneider T, Higgins DM, Botnar RM. Dark-blood late gadolinium enhancement without additional magnetization preparation. J Cardiovasc Magn R 2017;19.
Figures