0788
Free-breathing continuous cine and T1 mapping acquisition using a motion-corrected dual flip angle inversion-recovery spiral technique at 3 T1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 3Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 5Cardiology, Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
Synopsis
We propose a technique to obtain cine images and accurate B1-corrected T1 maps in a single free-breathing continuous Look-Locker inversion-recovery acquisition modified to use two excitation flip angles. Data are acquired using a single spiral interleaf, rotated by the golden-angle in time, with an inversion RF pulse applied every four seconds. Cine images are reconstructed from the steady state portion of the signal, while T1 mapping fits the model using maps with two flip angles. This strategy provides cine images and T1 maps, as well as a flip angle scale factor map, in a single free-breathing continuous acquisition.
Introduction
Cardiac magnetic resonance (CMR) cine imaging has become the “gold standard” to evaluate cardiac function, and T1 mapping has demonstrated the ability to assess both focal and diffuse myocardial processes in cardiomyopathy1,2. Clinically, these images are acquired in separate breath-holds with different sequences. In addition, T1 quantification methods that use continuous Look-Locker acquisition techniques are highly sensitive to B1 and slice profile effects3,4. To overcome these issues and simplify the acquisition, we propose a new strategy to obtain cine images and T1 maps, along with a flip angle (FA) scale factor map in a single free-breathing continuous inversion-recovery acquisition using two excitation flip angles.Method
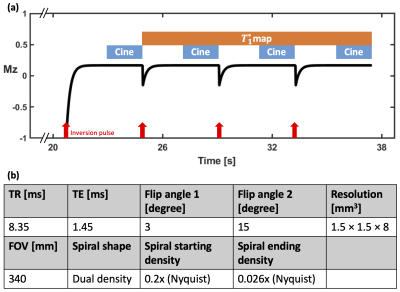
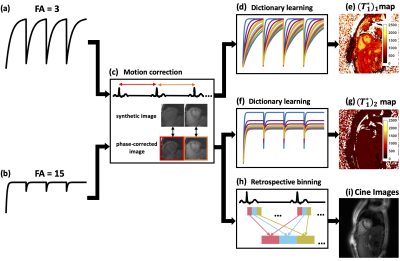
Acquisition strategyAs shown in Figure 1(a), following an inversion-recovery (IR) RF pulse, spiral trajectories were acquired continuously over 4 seconds using a gradient-echo (GRE) pulse sequence. This pattern was repeated 4 times with the first flip angle. After a 4-second relaxation period, the acquisition scheme was repeated using a second flip angle. The two flip angles (3° and 15°) were determined based on a Monte Carlo simulation (Figure 2). An ECG signal was used to determine the cardiac cycles. An automatic algorithm5,6 was used to detect the heart and select RF-coil array elements that had high SNR and minimal remote coil artifacts. As shown in Figure 3, principal component analysis (PCA) was performed on the phase-corrected images from each heartbeat to generate synthetic images for image registration to correct respiratory motion.
T1 mapping
For T1 mapping, every 5 spirals were combined to create a set of 4 images during a 167 ms diastolic acquisition window for each R-R interval. A dictionary was generated by k-SVD7 using Bloch simulation time courses with the acquisition parameters (Figure 1(b)) and T1 ranging from 200 to 3000 ms, first flip angle from 1° to 4°, second flip angle from 13° to 16°, and IR efficiency from 0.9 to 1. Dictionary learning8,9 reconstruction was performed by using Orthogonal Matching Pursuit (OMP) and Sparse Linear Equations and Sparse Least Squares (LSQR) to solve the equation:$$\underset{x, a_{p}}{\operatorname{argmin}}\|y-F S x\|_{2}^{2}+\lambda \Sigma_{n}\left\|R_{n}[x]-D a_{p}\right\|_{2}^{2} \quad \text { s.t. }\left\|a_{p}\right\|_{0} \leq K \quad [1]$$where $$$y$$$ is the acquired under-sampled k-space data, $$$F$$$ is the Fourier transform operator, $$$S$$$ is the coil sensitivity map, $$$x$$$ is the vectorized T1-weighted image to be reconstructed, $$$λ$$$ is the regularization parameter, $$$R_{n}$$$ is the operator to choose the nth pixel through time, $$$D$$$ is the dictionary, $$$a_{p}$$$ is the sparse representation of the pth curve with respect to $$$D$$$, and $$$K$$$ is the sparsity level. After reconstruction, images after the 2nd-4th IR and 5th-8th IR were fitted to a 3-parameter model:$$M(t)=M_{s s}-\left(M_{s s}+E_{I R} M_{s s}\right) e^{-t / T_{1}^{*}} \quad [2]$$where $$$M_{ss}$$$ is the signal after reaching steady state, $$$E_{IR}$$$ is the IR efficiency and $$$T_{1}^{*}$$$ is the apparent T1. Then based on the relationship between $$$T_{1}^{*}$$$ and T110, the T1 map can be solved using a set of two equations:$$\begin{array}{l}{\frac{1}{\left(T_{1}^{*}\right)_{1}}=\frac{1}{T_{1}}-\frac{\ln \cos \left(\beta F A_{1}\right)}{T R}}\quad [3]\\ {\frac{1}{\left(T_{1}^{*}\right)_{2}}=\frac{1}{T_{1}}-\frac{\ln \cos \left(\beta F A_{2}\right)}{T R}}\quad [4]\end{array}$$where $$$β$$$ is the scale factor between the nominal flip angle and the real applied flip angle, and the two apparent T1 maps $$${\left(T_{1}^{*}\right)_{1}}$$$ and $$${\left(T_{1}^{*}\right)_{2}}$$$ are obtained for two flip angles with Eq.[2]. Along with the T1 map, this fitting scheme also provides the flip angle scale factor ($$$β$$$) map.
Cine imaging
Cine images were reconstructed from the data where the signal is approaching steady state for FA2, which has better SNR than the ones under FA1. After retrospective ECG binning, cine images were reconstructed using Low rank plus Sparsity technique11.
Imaging experiments
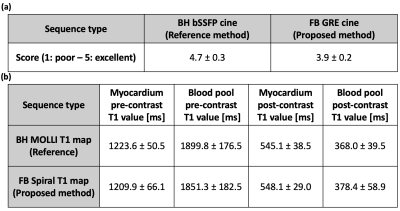
Imaging of T1MES phantom12 and 5 volunteers and patients were performed on a 3 T scanner (SIEMENS Prisma/Skyra). The clinical used breath-hold Cartesian bSSFP cine images and MOLLI13 T1 maps were also acquired as reference images. The T1 values were compared by drawing region of interests (ROIs) in the myocardium and blood pool. The cine image quality was graded on a 5-point scale (5: excellent, 1: poor) by an experienced cardiologist.
Results
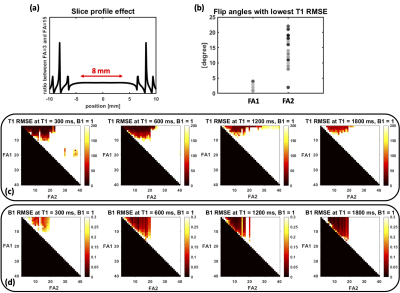
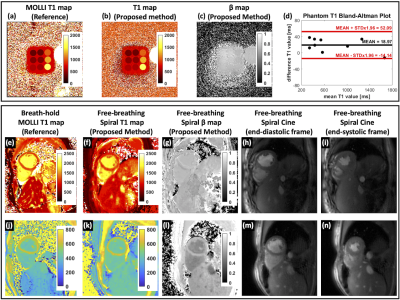
Figure 2(a) shows the slice profile ratio curve and verifies the assumption that the ratio of flip angles is linear for the chosen flip angles. The T1 and B1 normalized root-mean-squared-error (RMSE) map for different combinations of two flip angles is depicted in Figure 2(c)(d) for 4 typical T1 values in myocardium and blood pool with both pre- and post-contrast. Figure 2(b) summarizes the T1 RMSE as a function of flip angle and indicates the choice of the 2 flip angles: FA1 = 3° and FA2 = 15°. Figure 4 shows the image results for both phantom and human subjects. The phantom T1 map results from the proposed method are in close agreement with the MOLLI pulse sequence. The human studies demonstrate the high-quality cine images (Figure 5(a)) and T1 maps, along with comparable T1 quantifications as to MOLLI (Figure 5(b)).Discussion and Conclusion
We developed a new strategy to acquire cine images and T1 map in a single free-breathing, continuous inversion-recovery acquisition using duel excitation flip angles. T1 values from the proposed method are comparable to the standard breath-held MOLLI pulse sequence and the cine images show good image quality.Acknowledgements
This work was supported by NIH R01 HL131919, a Grant from the Coulter Foundation and AHA pre-doctoral fellowship.References
1. Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, Sibley CT, Bluemke D a. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14(1):90.
2. Jr ANA, Melo MDT De, Lima CR, Jr RND, Nomura CH, Salemi VMC, Jerosch-herold M, Parga JR. Myocardial T1 mapping and extracellular volume quantification in patients with left ventricular non-compaction cardiomyopathy. European Heart Journal - Cardiovascular Imaging. 2018;0:1–8.
3. Yarnykh VL. Optimal radiofrequency and gradient spoiling for improved accuracy of T1 and B1 measurements using fast steady-state techniques. Magnetic Resonance in Medicine. 2010;63(6):1610–1626.
4. Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: Searching for common ground. Magnetic Resonance in Medicine. 2015;73(2):514–522.
5. Zhou R, Huang W, Yang Y, Chen X, Weller DS, Kramer CM, Kozerke S, Salerno M. Simple motion correction strategy reduces respiratory-induced motion artifacts for k-t accelerated and compressed-sensing cardiovascular magnetic resonance perfusion imaging. Journal of Cardiovascular Magnetic Resonance. 2018;20(1):1–13.
6. Zhou R, Weller DS, Yang Y, Jacob M, Kramer CM, Ahmed AH. Free‐breathing cine imaging with motion‐corrected reconstruction at 3T using SPiral Acquisition with Respiratory correction and Cardiac Self‐gating (SPARCS). Magnetic Resonance in Medicine. 2019;00:1–15.
7. Aharon M, Elad M, Bruckstein A. K -SVD: An Algorithm for Designing Overcomplete Dictionaries for Sparse Representation. Signal Processing, IEEE Transactions on. 2006;54(11):4311–4322.
8. Ravishankar S, Bresler Y. MR image reconstruction from highly undersampled k-space data by dictionary learning. IEEE Transactions on Medical Imaging. 2011;30(5):1028–1041.
9. Zhu Y, Kang J, Duan C, Nezafat M, Neisius U, Jang J, Nezafat R. Integrated motion correction and dictionary learning for free-breathing myocardial T1 mapping. Magnetic Resonance in Medicine. 2019;81(4):2644–2654.
10. Deichmann R, Haase A, Hubland A. Quantification of Tl Values by SNAPSHOT-FLASH NMR Imaging. Journal of Magnetic Resonance. 1992;612:608–612.
11. Otazo R, Candès E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magnetic Resonance in Medicine. 2015;73(3):1125–1136.
12. Captur G, Gatehouse P, Kellman P, Heslinga FG, Keenan K, Bruehl R, Prothmann M, Graves MJ, Chiribiri A, Ittermann B, Pang W, Nezafat R, Salerno M, Moon JC. A T1 and ECV phantom for global T1 mapping quality assurance: The T1 mapping and ECV standardisation in CMR (T1MES) program. Journal of Cardiovascular Magnetic Resonance. 2016;18(S1):1–4.
13. Messroghli DR,
Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified
look-locker inversion recovery (MOLLI) for high-resolution T 1 mapping of the
heart. Magnetic Resonance in Medicine. 2004;52(1):141–146.
Figures