0787
Accelerated In Vivo Cardiac Diffusion Tensor MRI with Residual Deep Learning based Denoising in Lean and Obese Subjects1Cardiovascular Research Center, Massachusetts General Hospital, Charlestown, MA, United States, 2Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 3Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, University Medical Center Groningen, Groningen, Netherlands, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Department of Medicine, Harvard Medical School, Boston, MA, United States, 7Weight Center, Massachusetts General Hospital, Boston, MA, United States, 8Department of Surgery, Harvard Medical School, Boston, MA, United States
Synopsis
In vivo cardiac DT-MRI allows for imaging of the underlying myocardial fiber orientations but is hindered by clinically infeasible scan times. We developed and tested a residual deep learning denoising algorithm, DnCNN-54, on cardiac DT-MRI scans with fewer averages (4, 2, and 1) than the conventional 8-average 30 minute scan. We demonstrated a 2-fold acceleration can be achieved after DnCNN-54 is applied to 4 average dataset compared with the reference 8-average scan that preserves signal to noise ratio and cardiac DT-MRI parameter quantification. This 2-fold acceleration via DnCNN-54 denoising also maintained cardiac DT-MRI mean differences between obese and lean subjects.
INTRODUCTION
Obesity is key risk factor and precursor for cardiovascular disease such as heart failure and atrial fibrillation (1). Cardiac Diffusion Tensor Magnetic Resonance Imaging (DT-MRI) is capable of revealing underlying myocardial fiber orientations and microstructure. A main challenge with cardiac DT-MRI is the long scan time durations that can exceed 30 minutes due to the 8 averages obtained to result in a sufficiently high signal to noise ratio (SNR) with accurate cardiac DT-MRI parameter quantification (2-4). We aimed to reduce scan time by incrementally decreasing the number of averages and applying a residual deep learning algorithm (5) trained on in vivo cardiac DT-MRI data, called DnCNN-54, to reduce scan time without giving up SNR or parameter quantification.METHODS
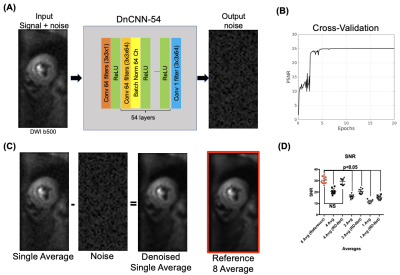
Unlike conventional deep learning generative algorithms, DnCNN-54 was trained to identify and then remove the residual noise image from the input image yielding the desired denoised image thereby avoiding overfitting and unknown failure modes (Figure 1). For training data, 1000 noise images (output of DnCNN-54) were collected and 1000 input images of DnCNN-54 were created from adding the 1000 noise images to reference 8-average DT-CMR datasets. DnCNN-54 was trained with a cross validation ratio of 80/20 (20 epochs, momentum = 0.9, learning rate = 0.1, L2 regularization = 10-4) using a single GPU (GTX 1080Ti, Nvidia, Santa Clara, CA) and custom implementation on MATLAB (MathWorks, Natick, MA). A cohort of 10 healthy IRB-consented volunteers were scanned with a conventional 8-average whole-heart DT-CMR (M2 spin echo EPI: TE = 75ms, 2.5x2.5x8mm3, 12 slices, b = 500 s/mm2, 12 directions) protocol on a 3T clinical MRI scanner (Siemens Prisma). Signal to noise ratio (SNR) and DT-CMR parameter quantification of mean diffusivity (MD), fractional anisotropy (FA) and helix angle transmurality (HAT) from 4, 2 and 1 averages with and without the application of DnCNN-54 were compared against the reference 8 averages. Inter-class correlation (R2) was calculated to test for agreement and similarity.RESULTS
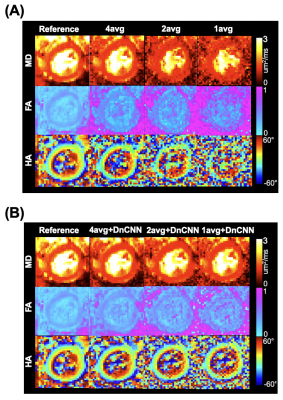
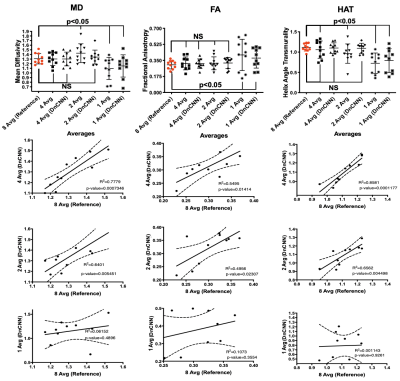
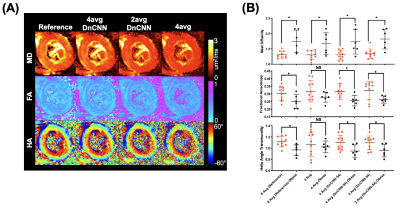
10 lean (6 male and 4 female, 26.5±3 years) and 6 obese study participants (5 female and one male, 46±11 years) were successfully scanned with in vivo DT-MRI. DT-MRI with 4 averages and DnCNN-54 provides comparable SNR (Figure 1B) and MD (R=0.7779, P<0.05), FA (R=0.5495, P<0.05) and HAT (R=0.8581, P<0.05 ) quantification (Figure 2 and 3) to the conventional 8-average DT-MRI scan leading to a 2-fold acceleration in scan time. In addition, DT-MRI with 2 averages and DnCNN-54 yielded comparable MD (0.6401, P<0.05), FA (R=0.4956, P<0.05) and HAT (R=0.6562, P<0.05) yielding a 4-fold acceleration in scan time but at the cost of significant decrease in SNR (19 ± 4 vs 31 ± 6) when compared with the reference 8-average DT-MRI scan. Mean differences in MD, FA, and HAT comparisons between obese and lean cohorts was only statistically significant (P<0.05) for reference 8-average (ΔMD=33.4%, ΔFA=-17.5%, ΔHAT=-13.0%), 4-average with DnCNN-54 (ΔMD=32.8%, ΔFA=-18.9%, ΔHAT=-13.7%), and 2-average DT-MRI with DnCNN-54 (ΔMD=35.2%, ΔFA=-18.3%, ΔHAT=-13.0%) (Figure 4).DISCUSSION
DnCNN-54 was applied to raw in vivo cardiac diffusion weighted MRI images to decrease noise and subsequently, tested to see if the number of acquired averages could be reduced to reduce overall scan time. DnCNN-54 applied to cardiac DT-MRI data using 4 averages resulted in comparable average SNR and cardiac DT-MRI parameter quantification with reference 8 average DT-MRI data representing a 2-fold acceleration in scan time. Significant correlation was found comparing MD, FA, and HAT of 4 averages scans with DnCNN-54 to the reference 8 average scans. In contrast without DnCNN-54, MD, FA, and HAT of 4 average scans did not significantly correlate with reference 8 average scans. Additionally, DnCNN-54 yielded comparable DT-MRI parameter quantification for 2 average data but did not preserve average SNR. MD correlations against the standard 8 average scans improved when DnCNN-54 was applied to the 2 average data but remains the same when correlations for FA and HAT were considered.Significant differences found between obese and lean subjects were preserved when acquiring 4 averages (accelerating by factor of 2) and 2 averages (acceleration by factor of 4) with DnCNN-54 for MD, FA, and HAT in comparison with reference 8 average data. However, 2 average data again exhibited significantly less SNR than the reference 8 average data. These significant differences between obese and lean subjects were completely lost when not applying DnCNN-54 for the 4, 2, and 1 average scans.
A limitation of this study is the small number of obese subjects to compare with lean subjects. Additional recruitment of obese subjects may change the exact differences found in this work and a larger cohort study is currently underway. However, we demonstrated DnCNN-54 faithfully preserves differences found between obese and lean groups despite the small number of subjects in half the scan time of a reference 8-average DT-MRI scan. This will more easily allow us to scan additional obese subjects given the accelerated DT-MRI acquisition.
CONCLUSION
We demonstrated that applying a residual deep learning denoising algorithm, DnCNN-54, can accelerate in vivo cardiac DT-MRI scans by a factor of 2 while still preserving SNR and DT-MRI parameter quantification. Furthermore, the detected DT-MRI parameter differences between obese and lean subjects were conserved when using DnCNN-54.Acknowledgements
NIH R21EB024701
Hassenfeld Scholar Award
MGH Spark Award
References
1. Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002 Aug 1;347(5):305–13.
2. Mekkaoui C, Reese TG, Jackowski MP, Bhat H, Sosnovik DE. Diffusion MRI in the heart. NMR Biomed. 2015 Oct 20;
3. Mekkaoui C, Reese TG, Jackowski MP, Cauley SF, Setsompop K, Bhat H, Sosnovik DE. Diffusion Tractography of the Entire Left Ventricle by Using Free-breathing Accelerated Simultaneous Multisection Imaging. Radiology. 2017 Mar;282(3):850–6. PMCID: PMC5318239
4. Nguyen C, Fan Z, Xie Y, Pang J, Speier P, Bi X, Kobashigawa J, Li D. In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magn. Reson. Med. John Wiley & Sons, Ltd; 2016 Nov;76(5):1354–63. PMCID: PMC5067209
5. Zhang K, Zuo W, Chen Y, Meng D, Zhang L. Beyond a Gaussian Denoiser: Residual Learning of Deep CNN for Image Denoising. IEEE Trans Image Process. 2017 Jul;26(7):3142–55.
Figures