0785
16-fold accelerated, single-shot late gadolinium enhancement CMR using GRASP for multi-TI reconstruction1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 3Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Division of Cardiology, Internal Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Synopsis
Late gadolinium enhanced (LGE) CMR is the gold standard test for assessment of myocardial scarring. There is unmet need for high resolution, free-breathing LGE CMR for patients with arrhythmia and/or dyspnea. The purpose of this study was to develop and clinically evaluate a high resolution, free-breathing LGE CMR sequence combined with radial k-space sampling and compressed sensing (CS), which enables image contrast optimization without a TI scout.
Introduction
Late gadolinium enhanced (LGE)(1-3) cardiovascular magnetic resonance (CMR) is the gold standard test for assessment of myocardial scarring. While single-shot LGE CMR is the preferred method for patients with arrhythmia and/or dyspnea, its relatively low spatial resolution (~2 mm x 3 mm) may reduce accuracy for visualizing small, subendocardial infarcts, quantifying myocardial scar volume, and identifying peri-infarct zones. One approach to achieve high spatial resolution with single-shot LGE is to accelerate it using compressed sensing (CS), but only at a moderate acceleration factor (2 or 3). In this study, we sought to exploit temporal redundancy in multi-frame (i.e. cine) information by achieving 16-fold acceleration using Golden-angle RAdial Sparse Parallel (GRASP) reconstruction with high spatial resolution (~ 1.3 mm x 1.3 mm) and evaluate its performance against clinical standard single-shot LGE in patients undergoing routine clinical CMR.Methods
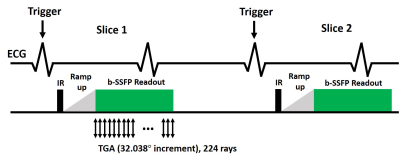
Human Subject: We enrolled 14 consecutive patients (mean age = 63.9 ± 18.6 years; 10 males; 4 females) undergoing clinical CMR at a 1.5 Tesla scanner (Avanto, Siemens) with 0.15-0.2 mmol/kg of gadobutrol. For this study, clinical standard single-shot LGE was performed approximately 10-15 min after administration of contrast agent, and our single-shot LGE was performed immediately after it.Pulse Sequence: As shown in Figure 1, the sequence continuously acquires data starting at mid diastole of the first heartbeat, immediately after the inversion pulse and 20 ramp-up pulses, through the early systole of the second heartbeat, and this unit is repeated for all slices thereafter. For our new LGE, the relevant imaging parameters included: FOV of 300 x 300 mm, matrix size of 224 x 224, spatial resolution = 1.3 mm x 1.3 mm, slice thickness of 8 mm, TR = 3.1 ms, TE = 1.6 ms, 224 rays per slice (rebinned into 14 rays per frame, 16 time frames) with 32.038º angular increments, single-shot readout duration = 694 ms, flip angle 45º, inversion time (TI) = 460 ms (minimum TI), effective triggering every 2nd heartbeat. For clinical single-shot LGE, the relevant imaging parameters include: FOV of 380 mm x 345 mm, matrix size of 176 x 128, spatial resolution = 2.2 mm x 2.7 mm, slice thickness of 6 mm, TI = 275-330 ms, flip angle 40º.
Image Reconstruction: Dynamic LGE data with 224 k-space rays were rebinned to 16 frames with 14 rays per frame and reconstructed using the GRASP [Feng] (4) framework, resulting in effective acceleration factor of 16 and temporal resolution of 43.4 ms. To accelerate the GRASP reconstruction on a GPU workstation (Tesla V100, NVIDIA, 32 GB memory), we applied coil compressing using principal component analysis (PCA)(5) to produce 8 virtual coils and used GPU-based Non-Uniform Fast Fourier Transform (NUFFT)(6). Compressed sensing (CS) part of GRASP was performed by enforcing sparsity in time with temporal total variation (TTV) and temporal PCA (TPCA) as two orthogonal sparsifying transforms. We used nonlinear conjugate gradient with back-tracking line search as the optimization algorithm with 30 iterations with normalized regularization weight 0.004 for TTV and 0.002 for TPCA. We empirically determined these weights as highest values that do not produce significant blurring artifacts on training datasets. Any residual noise-like artifacts were suppressed during post processing using block-wise, low-rank filtering with 2 iterations.
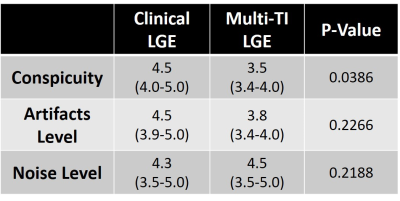
Reader scores: A total of 28 multi-slice LGE data sets (14 each for clinical and GRASP) were randomized and de-identified for visual analysis by two cardiovascular imaging attendings. Each multi-slice set was graded on a 5-point Likert scale (1: worst – 5: best) to evaluate: conspicuity of myocardial (or scar, if exists) border, artifact, and image noise, with 3 defined as clinically acceptable. We used the Wilcoxon signed-rank test to detect differences between two groups with average reader scores.
Results
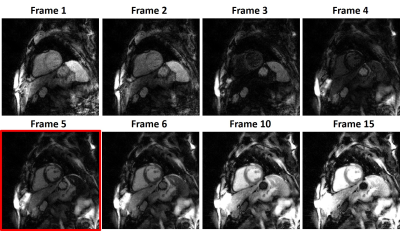
Figure 3 shows representative multi-TI LGE images for a single slice with different TIs, corresponding to different tissue contrast. Figure 4 shows representative clinical single-shot LGE images and multi-TI LGE images with the best TI for 4 different patients. The median conspicuity score was significantly different between the two groups, but both scores were greater than clinical acceptable (3.0) cut point. The median artifact and noise scores were not significantly different between the two groups.Discussions
This study demonstrates a novel single-shot LGE sequence with high spatial resolution and multi-TI information. Our approach allows for direct free-breathing scans without a TI scout and provides clinically acceptable image quality. The technique also has the potential to avoid poor image contrast due to incorrect TI, although this was not directly assessed in our current study. A future study includes automated identification of optimal TI. Factors that may have influenced the difference in conspicuity scores include: a) pulse sequence order, where multi-TI LGE (slightly less gadolinium) was acquired after the clinical LGE and b) slice thickness (6 mm for clinical vs. 8 mm for multi-TI LGE). Additional studies are warranted to further explore the potential of our multi-TI sequence to improve image quality, diagnostic confidence, and better optimize clinical workflow.Acknowledgements
This work was supported in part by the following grants: National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954) and American Heart Association (19IPLOI34760317).References
1. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100(19):1992-2002.
2. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343(20):1445-1453.
3. Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218(1):215-223.
4. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952-962.
5. Huang F, Vijayakumar S, Li Y, Hertel S, Duensing GR. A software channel compression technique for faster reconstruction with many channels. Magn Reson Imaging 2008;26(1):133-141.
6. Knoll F, Schwarzl A, Diwoky C, Sodickson DK. gpuNUFFT-an open source GPU library for 3D regridding with direct Matlab interface. In: Proceedings of the 22rd Annual Meeting of ISMRM, Melbourne, Australia 2014. Abstract No. 4297.
Figures