0779
AI-supported Segmentation of the Whole Left Atrium in Cine MRI Identifies a New Geometrical Predictor of Outcome in Atrial Fibrillation1Department for Radiology, University Hospital Basel, Basel, Switzerland, 2Department for Cardiology, University Hospital Basel, Basel, Switzerland
Synopsis
Deep learning based, automatic segmentation of the whole left atrium in cine MRI makes detailed geometrical analysis possible by fitting of an ellipsoid into the contours of the left atrium. Therefore, we could identify the ellipsoidal volume at the time-point before atrial contraction as an independent predictor of recurrence of atrial fibrillation after catheter ablation in a multivariable analysis.
Introduction
Atrial fibrillation (Afib) is a disease leading to severe complications like stroke, myocardial infarction, etc. and incidence is expected to rise over the next decade [1]. It causes anatomical changes of the left atrium (LA) like increase of LA size over time and outcome results worsen the longer Afib persists. Studies which show changes of LA geometry are in general based on single time-point analysis [2]. When aiming at analysing multiple time-points of the full cardiac cycle, time consuming LA segmentation is required. Hence, we trained a neural network to automatically segment the left atrium for further analysis and identify predictors of Afib recurrence after catheter ablation (CA).Methods
In our Afib database, 181 patients (pts) underwent pre-interventional MRI on a 1.5T scanner using a steady-state free precision cine sequence in transversal orientation with a slice thickness of 6 mm. A stack of the up to 12 slices was acquired to cover the whole left atrium, temporal resolution was 40 ms. All patients underwent catheter ablation after MRI. If they presented with recurrence, a second CA was allowed.We build a training set of 50 randomly selected patients. In those patients, the left atrium was semi-automatically segmented on all images, excluding the pulmonary veins and the appendage, using the freely available software Segment version 2.2 R6435 (http://segment.heiberg.se) [3]. This includes approx. 250 images per patient.
After imaging data was split into three dimensions in time, a 3D anisotropic U-Net architecture was implemented using an open-source, convolutional neural network for automatic segmentation of the LA [4].
After completion of training, all 181 Afib patients were processed with the trained model and spliced back. Those segmentations were each validated by visual inspection.
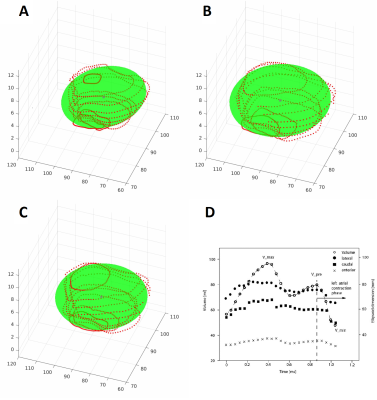
In a next step, an ellipsoid was fitted into the LA using a custom-written Matlab code (Matlab, MathWorks, USA) allowing for geometrical characterization of the LA (volume, surface area, x, y and z axes). We defined dimensions at three time-points for each patient (minimal value, maximal value and value before atrial contraction) [5, 6].
Regression analysis was performed and multivariable-adjusted Cox model was calculated to examine relations of those parameters, follow up data and recurrence of Afib after CA.
Results
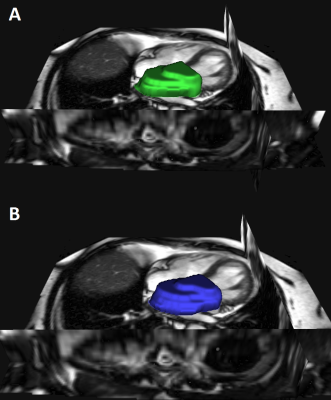
Semi-automatic segmentation of the LA took approx. 45-60 min/patient (Figure 1) whereas automatic segmentation with the built AI-algorithm needed approx. 30 sec to perform segmentation (Figure 2, Figure 3). During visual inspection of all segmentation, there was no need for editing of the AI-fitting but two patients were excluded. In total, automatic segmentation was feasible in 179/181 patients (98.9%). Dice similarity coefficient was 92±2% for separate test data (Figure 2). We excluded all patients with Afib during MRI (70 pts) and/or with prior left atrial CA (10 pts). Finally, 101 patients were included for further analysis (75% paroxysmal Afib).Mean follow-up was 408 (±338) days after last CA and 84% of patients were recurrence-free. The only baseline parameter which was significantly different between the groups was a shorter Afib duration in recurrence-free patients (44±62 months vs. 100±68 months, p<0.001).
Between fitted ellipsoids of recurrence and recurrence-free groups, significant differences were found in following ellipsoid parameters: maximal volume (116.6±24.7 ml vs. 98.7±31.6 ml, p=0.034), minimal volume (62.6±18.6 ml vs. 49.9±20.4 ml, p=0.022) and volume before atrial contraction (92.8±22.0 ml vs. 75.5±24.8 ml, p=0.011). In addition, minimal surface area (8444.7 mm2 vs. 7298.3 mm2, p=0.028), surface area before atrial contraction (10951.1 mm2 vs. 9624.2 mm2, p=0.016), minimal x axis (36.0±4.7 mm vs. 33.2±4.8 mm, p=0.045) and minimal z axis (15.2±2.9 mm vs. 13.5±3.1 mm, p=0.041) were also significantly different.
In a multivariable Cox model (adjusted for age, gender, BMI and duration of Afib), the LA volume before atrial contraction (in ml) of the ellipsoid was identified as an independent predictor for Afib recurrence (HR 1.036, CI 1.008-1.064, p=0.011).
Discussion
Semi-automatic segmentation of the LA in cine images is a time consuming procedure. Opposite to that, our AI-based segmentation tool for the LA in patients with Afib is very fast and reliable. Further fitting of an ellipsoid in the segmented LA makes a detailed analysis of LA geometry and function possible. Thereby, we were able to analyse almost 180 exams of our database and could identify a new predictor for recurrence after catheter ablation in Afib patients in a multivariable analysis.Its use might be limited to Afib patients since it was trained on an Afib collective but this is feasible and time-efficiency outweighs this limitation in our view. Despite there was no necessity for manual editing of AI segmentation in our processed cases, visual inspection is mandatory in every case to check for plausibility.
Conclusion
Fully automatic, deep learning derived segmentation of the whole LA based on cine MRI of Afib patients is feasible and enables geometrical analysis by fitting of an ellipsoid. Thus, LA ellipsoidal volume before atrial contraction could be identified as an independent predictor for recurrence after CA. This finding could be used for patient selection prior to catheter ablation to support clinical decision making and improve patient care.Acknowledgements
No acknowledgement found.References
1. Rahman, F., G.F. Kwan, and E.J. Benjamin, Global epidemiology of atrial fibrillation. Nat Rev Cardiol, 2014. 11(11): p. 639-54.
2. Kato, R., et al., Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation, 2003. 107(15): p. 2004-10.
3. Heiberg, E., et al., Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging, 2010. 10: p. 1.
4. Çiçek, Ö., et al. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. 2016. Cham: Springer International Publishing.
5. Kowallick, J.T., et al., Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson, 2014. 16: p. 60.
6. Leng, S., et al., Validation of a rapid semi-automated method to assess left atrial longitudinal phasic strains on cine cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson, 2018. 20(1): p. 71.
Figures